Manuscript accepted on :06 Sep 2019
Published online on: 28-09-2019
Plagiarism Check: Yes
Reviewed by: Lobna Sherif
Second Review by: Amal Hassanain
Final Approval by: Prof. Juei-Tang Cheng
Maged A. El Wakeel1, Manal A. Shehata1*, Ghada M. El-Kassas1, Hend H. Mostafa1, Essam M. Galal1, Salwa Refat El-Zayat2 and Nagwa Abd EL Ghaffar Mohammed3
1Department of Child Health, National Research Centre, Cairo, Egypt.
2Medical physiology department National Research Centre, Cairo, Egypt.
3Clinical and chemical pathology department National Research Centre, Cairo, Egypt.
Corresponding Author E-mail: dr_manalabdelkader@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1766
Abstract
Childhood obesity has been linked to an increase in fracture risk, so the impact of obesity on bone metabolism is becoming a focus of attention to identify factors that may affect bone health in obese children. Therefore, this study aimed to examine the association between serum 25-Hydroxy vitamin D [25(OH) D], adipokines and bone status in obese children. This case control study was executed in the Child Health Clinic in Medical and Scientific Centre of Excellence, National Research Centre (NRC), 100 obese and 80 non-obese age- and sex-matched children were enrolled in our study with mean age of (10.12±2.34 & 9.62±1.67 years) respectively. Anthropometric measurements, femoral neck bone mineral density (BMD) and its Z-score, bone mineral content (BMC) were measured using dual-energy X-ray absorptiometry (DXA) in relation to body weight (kg), we also determined serum 25(OH) D, adiponectin, leptin and lipid profile. HOMA-IR was calculated to assess insulin resistance. It was found that BMC and BMD Z-score adjusted for weight were significantly lower in obese children as compared to controls (all p<0.05). Obese children had lower levels of 25(OH) D and adiponectin (P<0.01), while higher levels of leptin, total cholesterol (TC) and triglycerides (TG) compared to controls (P<0.01). Both BMC and BMD Z-score showed positive association with 25(OH) D and adiponectin (P<0.01) and negative association with HOMA-IR, TG and TC (P<0.05). Linear regression analysis showed that 25(OH) D was the most effective factor predicting BMD Z-score and BMC in obese children. It is concluded that, obesity is negatively related to bone health in childhood. Those obese children are at increased risk for vitamin D insufficiency, which shows an obvious relationship to lower bone mass, raising the question of supplementation to prevent the deleterious effect of its deficiency on bones and reducing future risk of fracture and osteoporosis.
Keywords
Adipokines; Bone Health; Childhood Obesity; Serum 25(Oh) D
Download this article as:| Copy the following to cite this article: Wakeel M. A. E, Shehata M. A, Kassas G. M. E, Mostafa H. H, Galal E. M, Zayat S. F. E, Mohammed N. A. E. G. Bone Health in Relation to Vitamin-D Status And Serum Adipokines in Obese Egyptian Children. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Wakeel M. A. E, Shehata M. A, Kassas G. M. E, Mostafa H. H, Galal E. M, Zayat S. F. E, Mohammed N. A. E. G. Bone Health in Relation to Vitamin-D Status And Serum Adipokines in Obese Egyptian Children. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28594 |
Introduction
Increasing recrudescence of childhood obesity is a crucial public health concern.1,2 The vast predominance of childhood overweight or obesity is in developing countries, with above 30% increased rate higher than that of developed countries.3 Some studies have demonstrated a counter association of obesity with bone mineral density and bone health in children and adolescents .4,5,6 Along with the higher risk of sustaining fractures which are more complex in obese children as compared to normal weight peers.4,7
Vitamin D has a significant role in bone growth, obese children have been found to possess lower concentration of vitamin D 8,9, that may contribute to lower BMD, as suggested by some studies.6,10
Adipokines are adipose tissue secreted cytokines, playing a major role in regulating metabolic processes, from which leptin and adiponectin have a major role in obesity-related comorbidities and complications.11 both of them have been found to perform both a direct and centrally mediated influence on skeletal metabolism and development.12
Leptin, an adipocyte-derived hormone known for its role in energy homeostasis, has also been suggested to play a major role in bone metabolism, via its direct influence on skeletal tissue and concomitantly thorough modulating the bone-regulating hormones.13,14 The fat amount and thence leptin concentration, possess a dose-mediated influence on skeletal metabolism; little concentrations of leptin represent an osteoprotective effect, but at higher concentrations, bone wastage is augmented by bone resorption and slashed bone formation.12
Adiponectin, the most prevailing adipo-cytokine in plasma, which implicated in glucose regulation and fatty acid breakdown and have a protective effect in various processes such as energy metabolism, inflammation and cell proliferation.15 An effective role of adiponectin in bone metabolism with a counter relation between adiponectin and bone has been brightened up.16
There is a highlighted interaction between insulin resistance and bone health, which may be interposed by visceral adiposity and the levels of circulating insulin.17
The aim of this study was to assess the impact of obesity on bone metabolism and to clarify the role of vitamin D status, adipokines and other factors that may affect bone health in Egyptian obese children.
Materials and Methods
This was a case-control study, enrolling one hundred obese children compared to a control group of age and sex matched eighty apparently healthy non-obese children, recruited from the child health Clinic in Medical and Scientific Centre of Excellence, National Research Centre (NRC). The study was approved by the Medical Ethical Committee of the National Research Centre, Cairo, Egypt. A written informed consent was obtained from parents of all participants after explanation of the objectives of the study.
Involved children fulfilled the inclusion criteria of simple obesity with age ranged between 5-15 years from both sexes who accepted to participate in the study, excluding children with endocrinal or genetic causes of obesity, chronic debilitating diseases that may interfere with bone health such as: e.g. (renal or hepatic disease), or mal-absorptive disorders (Crohn’s disease, celiac disease and cystic fibrosis) and cancer. Children taking medications such as systemic glucocorticoids or anticonvulsants or those taking vitamin D, calcium or multivitamin supplements.
All enrolled children in the study were subjected to careful history taking, thorough clinical examination and anthropometric measurements; weight and height were measured in light clothing with no shoes. Body mass index (BMI) was calculated as weight (kg)/height (m2), Weight for age, height for age and BMI Z-score were calculated with the help of Anthro-plus Program for personal computers based on the WHO growth standards 18, obesity was considered with BMI- Z-score >2. Triceps skinfold (SFT) was obtained by measuring the mid-point between the tip of the shoulder and the tip of the elbow (olecranon process and the acromium), parallel to the long axis of a slightly flexed arm, using the Holtain skinfold calipers, the circumference of the left upper arm, at the same mid-point giving the mid-arm circumference (MAC). Waist circumference at the smallest point between the rib cage and the iliac crest, Hip circumference at the largest width over the greater trochanters (the widest diameter around the buttocks), all of them have been measured with a non-elastic flexible tape and recorded to the nearest 0.1 cm, waist / Hip ratio (W/H R) was then calculated.
Laboratory Determinations
After fasting for 10-12 hours, venous blood sample was taken and left to clot, centrifuged, Sera were separated and stored at –20 ° C until assay.
Fasting serum glucose, Cholesterol, Triglycerides (TG), low density lipoprotein (LDL) and high-density lipoprotein (HDL) were measured by enzymatic calorimetric method using Bio-diagnostic kit (Egypt)
The homeostasis model (HOMA-IR) was calculated according to the known formula [fasting insulin (mIU/ml) × fasting glucose (mg/dl)/405]. Insulin resistance has been considered if HOMA-IR index > 3.16, the greater the HOMA-IR value, the greater insulin resistance degree.
Serum levels of insulin, adiponectin, Leptin and vitamin D were measured by a solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle, using Enzyme immunoassay kit of insulin (Chemux Bioscience, Inc, USA), adiponectin (Assay pro, USA), leptin (Diagnostic Automation/Cortez Diagnostics, Inc. USA) and vitamin D following instructions of the kits purchased from (Epitope Diagnostic, Inc. San Diego, CA 92121, USA).
Measurement of bone mineral content (BMC) in grams, bone mineral density (BMD) in (g/cm2), bone area (cm2) was done by trained radiology technologist using dual energy X-ray absorptiometry (DXA) (Norland Capital- XR46; Norland Medical systems Inc., Fort Atkinson, WI) according to the procedures recommended by the manufacturer. Measurements were taken in separate regions of interest: femur neck, and lumbar spine (L1–L4).
Statistical Analysis
All data were collected, verified, coded, entered and analyzed using IBM SPSS Statistics v. 22. Comparison between two groups regarding quantitative data was done using Independent t-test. DXA, bone parameters values were compared after adjustment for total body weight using a 1-way analysis of covariance. The relation between two quantitative parameters in the same group was assessed by Pearson correlation analysis. Linear regression analysis was performed to identify possible determinants associated with the Z-scores for BMD and BMC after adjusting for effects of weight. P-value <0.05 was considered significant.
Results
The present study enrolled 100 obese and 80 non-obese age- and sex-matched Egyptian children with mean age (10.12±2.34 & 9.62±1.67 years), respectively. Obese children had statistically significant higher anthropometric indices (weight Z-score, BMI Z-score, Triceps skinfold, mid-arm circumference and waist / Hip ratio, P < 0.05 for all) than non-obese group. After adjustment for body weight, BMC and BMD Z-score at femoral neck (FN) and lumbar vertebra (LV2-LV4) were significantly lower in obese children (all p<0.05), while no difference in BMD at any site of analysis as compared with normal-weight children (Table 1).
Table 1: demographic data, anthropometric and bone health indices of obese children versus controls.
| Variables | Obese group
n=100 Mean ±SD |
Control group
n=80 Mean ±SD |
p-value |
| Age (years) | 10.12±2.34 | 9.62±1.67 | 0.175 |
| Weight Z-score | 2.64±0.93 | 0.9684±0.22 | <0.001* |
| Height Z-score | -0.31±0.69 | -0.70±0.72 | 0.656 |
| BMI Z-score | 2.67±0.82 | 1.10±0.47 | <0.001* |
| Skin fold (mm) | 20.60±4.78 | 10.1±2.58 | <0.001* |
| waist / Hip ratio | 0.90±0.1 | 0.76±0.2 | 0.04* |
| Mid-arm (cm) | 36.40±8.71 | 20.3±4.1 | <0.001* |
| BMD.FN (g/cm2) | 0.76±0.16 | 0.80±0.17 | 0.33 |
| BMC.FN (g) | 3.61±0.98 | 4.22±0.74 | 0.004* |
| BMD.FN. Z-score | -0.18±0.61 | 0.35±0.85 | 0.002* |
| BMD.LV (g/cm2) | 0.65±0.17 | 0.58±0.13 | 0.067 |
| BMC.LV (g) | 35.07±7.11 | 38.72±4.29 | 0.013* |
| BMD.LV. Z-score | -0.40±0.71 | 0.02±0.8 | 0.016* |
*(P ≤ 0.05): obese significantly different from controls
BMC: bone mineral content; BMD: bone mineral density; BMI: Body mass index; FN: femoral neck; LV: lumber vertebra; SD: Standard deviation.
Table (2) shows that After adjustment for body weight, obese children had lower levels of vitamin D (25(OH) D), adiponectin and high density lipoprotein (HDL) (P<0.01), while higher levels of leptin, total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL) & HOMA-IR compared to controls (P<0.01).
Table 2: laboratory features of obese children versus controls.
| Variables | Obese group
n=100 Mean±SD |
Control group
n=80 Mean±SD |
p-value |
| 25(OH) D (ng/mL) | 10.13±3.22 | 17.68±11.64 | <0.001* |
| Cholesterol | 189.74±47.98 | 89.52±17.1 | <0.001* |
| Triglycerides | 131.63±34.36 | 81.68±24.76 | <0.001* |
| HDL | 49.28±11.6 | 58.26±11.21 | <0.001* |
| LDL | 129.40±28.42 | 47.94±10.62 | <0.001* |
| Adiponectin (ng/mL) | 37.4±8.5 | 54.7±11.4 | 0.03* |
| Leptin (ng/mL) | 41.34±11.3 | 4.7±2.4 | ˂0.001* |
| HOMA.IR | 5.12±1.34 | 1.85±0.6 | 0.001* |
* p<0.05 the relation is statistically significant
25(OH) D: 25-Hydroxy vitamin D; HOMA-IR: homeostasis model assessment of insulin resistance, LDL: low-density lipoprotein; HDL: high-density lipoprotein; SD: Standard deviation.
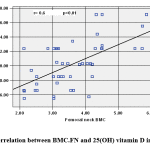 |
Figure 1: Correlation between BMC.FN and 25(OH) vitamin D in obese cases |
BMC.FN showed positive association with 25(OH) D (Figure 1) and negative association with Wt. Z-score (r=-.306, p=.031), BMI. Z-score (r= -0.291, p=0.04), Cholesterol (r= -0.373, p=0.008) (Figure 2), HDL (r= -0.513, p=0.000) & HOMA-IR, (r=-0.286, p= 0.044).
BMD.FN. Z-score showed positive association with 25(OH) D (r=0.376, p=0.007) & Adiponectin (r= 0.367, p=0.009) (figure 3) and negative association with TG (r=-0.327, p=0.020), Cholesterol (r= -0.295, p= 0.037) HDL (r= -0.474, p=0.001) & HOMA-IR (r=-.350, p=.013).
BMD.LV. Z-score showed positive association with 25(OH) D (r= 0.298, p=0.036) & Adiponectin (r= 0.337, p= 0.017) and negative association with HDL (r= -0.283, p=0.046) & HOMA-IR (r= – 0.312, p=0.027). Leptin was insignificantly correlated to bone variables in obese cases.
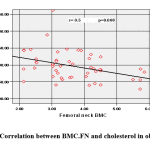 |
Figure 2: Correlation between BMC.FN and cholesterol in obese cases. |
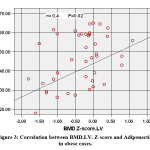 |
Figure 3: Correlation between BMD.LV. Z-score and Adiponectin in obese cases. |
Linear regression analysis showed that 25(OH) D was the most effective factor predicting BMC.FN (P<0.001) and BMD.FN. Z-score (P=0.05) in obese children (Table 3 & 4).
Table 3: Linear regression analysis for independent predictors of BMC.FN among the studied obese children.
| Predictors
|
BMC.FN | |
| Standardized CoefficientsBeta |
Sig. | |
| (Constant) | 0.006 | |
| 25(OH) D | 0.542 | <0.001* |
| Cholesterol | -0.147 | 0.283 |
| Triglycerides | -0.062 | 0.652 |
| Adiponectin | 0.002 | 0.990 |
| HOMA.IR | -0.054 | 0.690 |
* p<0.05 the relation is statistically significant
25(OH) D: 25-Hydroxy vitamin D; HOMA-IR: homeostasis model assessment of insulin resistance, BMC: bone mineral content; FN: femoral neck.
Table 4: Linear regression analysis for independent predictors of BMD.FN. Zscore among the studied obese children.
| Predictors | BMD.FN. Z-score | |
| Standardized CoefficientsBeta |
Sig. | |
| (Constant) | 0.260 | |
| BMI z-score | 0.155 | 0.327 |
| 25(OH) D | 0.299 | 0.05* |
| Cholesterol | -0.097 | 0.526 |
| Triglycerides | -0.114 | 0.467 |
| Adiponectin | 0.246 | 0.094 |
| HOMA.IR | -0.159 | 0.317 |
* p<0.05 the relation is statistically significant
BMD: bone mineral density; BMI: Body mass index; FN: femoral neck, 25(OH) D: 25-Hydroxy vitamin D; HOMA-IR: homeostasis model assessment of insulin resistance.
Discussion
This study was conducted to test the hypothesis that obesity is a risk factor for poor bone health in children and adolescents. After adjustment for body weight, BMC and BMD. Z-score were significantly lower in obese children (p<0.05), compared to control group. While the correlation analysis revealed an inverse association between BMC.FN and BMI Z-score. Conflicting results have been reported in several studies, regarding the relation between bone health and obesity. In close agreement, with our findings, a large scale national survey of U.S. young population from the NHANES found that both total body BMD and lumbar spine BMD had an inverse relation with degree of obesity, the authors suggested regional differences in the relation between adiposity and BMD.19 Another cross sectional study conducted on Indian children and adolescents found that total body BMC, bone area and BMD adjusted for Tanner stage and weight were significantly lower in obese children relative to overweight and normal weight children.4
However in contrast to these findings, other studies confirmed a positive association between BMD and fat mass.20 Similarly, Kim et al.21 evidenced a positive impact of fat mass and lean mass on bone density in Korean adolescents. This could match the findings of a recent meta-analysis about the comparison of BMD between obese or overweight children and controls, which found a significant higher BMD in the obese group.22
In the same context, the increased adiposity has traditionally been accounted to have a beneficial effect on bone status, as the mechanical loading was found to be a potent enhancer of the proliferation of osteoblasts and increased bone strength.23 Thus, this can partly explain the positive relation between adiposity and bone status. However, after adjustment for body weight, this positive association is no longer significant, 24 or is completely absent.25
Hence, it was indicated that the positive influence of body weight on BMD could not counteract the deleterious effects of obesity on bone health. However, the precise mechanism implying for deteriorated bone status in the obese is not completely elucidated yet and further studies are required.26
The influence of vitamin D as one of the most important bone-regulators is well-known, furthermore, accumulating evidences indicated the contribution of vitamin D to lower BMD.27 Simultaneously, serum concentrations of vitamin D had evidenced to be decreased in obese individuals 28, and inversely associated with BMI.7 These findings match our results as we observed a significant lower levels of vitamin D in obese subjects compared to control group. In addition, a negative association was found between vitamin D and BMI Z-score, BMC.FN and BMD.FN Z-score. Vitamin D also was a strong predictor of BMC.FN and for BMD.FN Z-score by linear regression in this study.
Recently it was suggested that decreased serum vitamin D is an outcome of obesity and the findings from the clinical trials that explored the association between vitamin D and obesity, are still infrequent and inconclusive. On the other hand, it was evidenced that vitamin D prevents fat deposition, promotes insulin synthesis, and reduces insulin resistance and hunger, that favor controlling obesity and T2DM.29
The contribution of some adipokines in bone remodeling had been considered as another probable mechanism. These molecules are released from fat cells and some of them interfere with both bone formation and resorption.30
Existed data regarding the role of leptin was inconsistent, Rhie and his colleagues 31, suggested that it plays a beneficial role on bone mass and density in prepubertal girls.
However, higher concentrations of leptin, had been proposed to stimulate inflammatory cascades in osteoblasts that may contribute to poor bone health.32
Although obese subjects in this study showed significantly higher levels of leptin, there was no significant correlation with any bone variables.
In accordance, in a previous review study, leptin was not associated with BMD after adjustment for BMI and fat mass in variable studies.33 The finding of another recent review may explain this controversy, as they reported that leptin can improve bone health in cases with deficient leptin blood levels, otherwise it is probably had no significant effect when it is not deficient.13
On the other hand, the role of adiponectin as a mediator of the relation between bone health and obesity was investigated, there was a direct association between BMD FN.Z-score and adiponectin, while its correlations with other bone health indices were insignificant, which match the finding of a previous longitudinal study, conducted on 96 adolescent healthy males.34
In contrast with the current study, the majority of the clinical studies, which had been discussed in a recent review, reported a negative association between adiponectin and bone health in populations with different age groups. However, most of in vitro studies that searched its signaling and mechanisms of actions partially supported our findings and revealed that the activities of adiponectin predicted a positive effect on bone, as it enhanced the proliferation and differentiation of osteoblasts. Moreover, the indirect effect of adiponectin was considered, through modulation of the sympathetic tone and the regulation of insulin sensitivity and energy homeostasis.35
Similarly, China et al. 36 reported that adiponectin positively influenced skeletal health, and they attributed the discrepancy found in literatures to the use of various structural forms of adiponectin in preclinical research.
In another respect, this study suggested the potential contribution of the metabolic disturbances associated with obesity to poor bone health as there were inverse associations between BMD.FN. Z-score with TG, Cholesterol, HDL & HOMA-IR. Furthermore, BMD.LV. Z-score showed negative association with HOMA-IR.
In accordance, the interrelation between dyslipidemia and low mineral density of bone was evidenced in several studies; elevated cholesterol had been proved a negative regulator of bone.37
In addition, HOMA-IR was used to evaluate insulin resistance in a study conducted by Pollock et al. 38; they suggested the contribution of insulin resistance in the link between obesity and low bone mass. Moreover, vitamin D concentrations were inversely associated with insulin resistance in the present study, that match other findings.39, 40
Furthermore, in agreement with our findings it was indicated that disturbed glucose regulation negatively affected the growing skeleton. However, the exact mechanism needs more elucidation.41
The major limitation of this study is being a cross-sectional one, thus studying the causal relations could not be inferred. Further longitudinal studies are needed, on larger sample size for better understanding of the complex situation.
Conclusion
In conclusion, rising concerns regarding the relation between childhood obesity and bone health have emerged nowadays. The current study demonstrated a significantly lower value of most of the studied bone health indices in obese children relative to controls, and a negative association was found between bone mineral density at femoral neck and BMI. Vitamin D represented the most effective predictor of bone health, indicating its important role in the mechanism implicated in mal-affecting bone status in obese. These findings may contribute in enhancing the preventive and therapeutic strategies to counteract the linked risks of obesity and poor bone health.
Acknowledgment
The authors would like to acknowledge the staffs of Medical and Scientific Centre of Excellence, National Research Centre, and all individuals who were directly and indirectly involved in this study.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding
This was a part of a research project, funded by National Research Centre 10th research plan. (Grant No. 11010144).
References
- Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review. Obesity reviews. 2018 Apr; 19(4):452-63.
- El Kassas GM, Shehata MA, El Wakeel MA, Amer AF, Elzaree FA, Darwish MK, Amer MF. Role of Procalcitonin As an Inflammatory Marker in a Sample of Egyptian Children with Simple Obesity. Open access Macedonian journal of medical sciences. 2018 Aug 20;6(8):1349.
- World Health Organization. Facts and figures on childhood obesity. Commission on ending childhood obesity. [Cited 2017]. Available from URL: https://www.who.int/end-childhood-obesity/facts/en/
- Khadilkar A, Chiplonkar S, Agrawal DP, Sanwalka N, Khadilkar V. Bone Health Status in Indian Overweight/Obese Children. The Indian Journal of Pediatrics. 2016 Dec 1;83(12-13):1473-5. https://doi.org/10.1007/s12098-016-2179-y
- El Wakeel MA, El-Kassas GM, Hashem SA, Abouelnaga MW, Elzaree FA, Hassan M, Abdelrahman AH, Mohammed NA. Potential role of oxidative stress in childhood obesity and its relation to inflammation. BIOSCIENCE RESEARCH. 2018 Oct 1;15(4):3791-9.
- Soininen S, Sidoroff V, Lindi V, Mahonen A, Kröger L, Kröger H, Jääskeläinen J, Atalay M, Laaksonen DE, Laitinen T, Lakka TA. Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6–8 years of age–The Physical Activity and Nutrition in Children (PANIC) study. Bone. 2018 Mar 1;108:106-14.
- Fornari ED, Suszter M, Roocroft J, Bastrom T, Edmonds EW, Schlechter J. Childhood obesity as a risk factor for lateral condyle fractures over supracondylar humerus fractures. Clinical Orthopaedics and Related Research®. 2013 Apr 1;471(4):1193-8.
- Abu Shady MM, Youssef MM, Shehata MA, Salah El-Din EM, ElMalt HA. Association of Serum 25-Hydroxyvitamin D with Life Style and Dietary Factors in Egyptian Prepubescent Children. OA Maced J Med Sci. 2015 Mar 15; 3(1):80-84. http://dx.doi.org/10.3889/oamjms.2015.006
- El-Shaheed AA, Sallam SF, El-Zayat SR, Sibaii H, Mahfouz NN, Moustafa RS, Ibrahim SM. Vitamin D level in children and its relation to immunity and general health condition. BIOSCIENCE RESEARCH. 2017 Apr 1;14(2):143-8.
- Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria Pantano A, Napoli N, Pozzilli P, Manfrini S. BMI and BMD: the potential interplay between obesity and bone fragility. International journal of environmental research and public health. 2016 Jun;13(6):544.
- El Wakeel MA, El-Kassas GM, Kamhawy AH, Galal EM, Nassar MS, Hammad EM, El-Zayat SR. Serum Apelin and Obesity-Related Complications in Egyptian Children. Open Access Maced J Med Sci. 2018 Aug 20; 6(8):1354-1358. https://doi.org/10.3889/oamjms.2018.312
- Dimitri P. Fat and bone in children–where are we now?. Annals of pediatric endocrinology & metabolism. 2018 Jun;23(2):62.
- Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015 Jan 1;64(1):105-13.
- Kouda K, Ohara K, Fujita Y, Nakamura H, Tachiki T, Iki M. Relationships between serum leptin levels and bone mineral parameters in school-aged children: a 3-year follow-up study. Journal of bone and mineral metabolism. 2019 Jan 25;37(1):152-60.
- Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed research international. 2014;2014.
- Jin J, Wang Y, Jiang H, Kourkoumelis N, Renaudineau Y, Deng Z. The impact of obesity through fat depots and adipokines on bone homeostasis. AME Medical Journal. 2018 Dec 1;3(1).
- Ma C, Tonks KT, Center JR, Samocha‐Bonet D, Greenfield JR. Complex interplay among adiposity, insulin resistance and bone health. Clinical obesity. 2018 Apr;8(2):131-9.
- World Health Organization. WHO AnthroPlus for personal computers Manual: Software for assessing growth of the world’s children and adolescents. Geneva: WHO, 2009 (http://www.who.int/growthref/tools/en/).
- Suárez GC, Singer BH, Gebremariam A, Lee JM, Singer K. The relationship between adiposity and bone density in U.S. children and adolescents. PLoS One. 2017;12:e0181587.
- Maggio AB, Belli DC, Puigdefabregas JW, Rizzoli R, Farpour-Lambert NJ, Beghetti M, McLin VA. High bone density in adolescents with obesity is related to fat mass and serum leptin concentrations. Journal of pediatric gastroenterology and nutrition. 2014 Jun 1;58(6):723-8.
- Kim HY, Jung HW, Hong H, Kim JH, Shin CH, Yang SW, Lee YA. The role of overweight and obesity on bone health in Korean adolescents with a focus on lean and fat mass. J Korean Med Sci. 2017;32:1633–1641.
- van Leeuwen J, Koes BW, Paulis WD, van Middelkoop M. Differences in bone mineral density between normal-weight children and children with overweight and obesity: a systematic review and meta-analysis. Obes Rev. 2017;18:526–546.
- Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annual review of biomedical engineering. 2006;8:455–98.
- Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54.
- Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–7.
- Shapses SA, Pop LC, Wang Y. Obesity is a concern for bone health with aging. Nutr Res. 2017;39:1–13. doi:10.1016/j.nutres.2016.12.010
- Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. The American journal of clinical nutrition. 2007 Nov 1;86(5):1530-8.
- Rodriguez-Rodriguez E, Navia-Lomban B, Lopez-Sobaler AM, Ortega RM. Associations between abdominal fat and body mass index on vitamin D status in a group of Spanish schoolchildren. European journal of clinical nutrition. 2010 May;64(5):461.. Eur J Clin Nutr. 2010;64:461–7.
- Cândido FG, Bressan J. Vitamin D: link between osteoporosis, obesity, and diabetes? Int J Mol Sci. 2014 Apr 17;15(4):6569-91. doi: 10.3390/ijms15046569. PubMed PMID: 24747593; PubMed Central PMCID: PMC4013648.
- Mosca L, da Silva V, Goldberg T. Does excess weight interfere with bone mass accumulation during adolescence?. Nutrients. 2013;5(6):2047-61.
- Rhie YJ, Lee KH, Chung SC, Kim HS, Kim DH. Effects of body composition, leptin, and adiponectin on bone mineral density in prepubertal girls. J Korean Med Sci. 2010;25:1187–1190.
- Yang WH, Tsai CH, Fong YC, Huang YL, Wang SJ, Chang YS, Tang CH. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. International journal of molecular sciences. 2014 Sep 5;15(9):15778-90.
- Biver E, Salliot C, Combescure C, Gossec L, Hardouin P, Legroux-Gerot I, Cortet B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2703–2713.
- Vaitkeviciute D, Lätt E, Mäestu J, Jürimäe T, Saar M, Purge P, Maasalu K, Jürimäe J. Longitudinal associations between bone and adipose tissue biochemical markers with bone mineralization in boys during puberty. BMC pediatrics. 2016 Dec;16(1):102. http://dx.doi.org/10.1186/s12887-016-0647-1.
- Naot D, Musson DS, Cornish J. The activity of adiponectin in bone. Calcified tissue international. 2017 May 1;100(5):486-99.
- China SP, Sanyal S, Chattopadhyay N. Adiponectin signaling and its role in bone metabolism. Cytokine. 2018 Dec 1;112:116-31.
- Mandal CC. High Cholesterol Deteriorates Bone Health: New Insights into Molecular Mechanisms. Front Endocrinol (Lausanne). 2015;6:165. doi:10.3389/fendo.2015.00165.
- Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J. Pediatr. 2011;158(5):727–734.
- Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33(10):2238–2243.
- Parikh S, Guo DH, Pollock NK, Petty K, Bhagatwala J, Gutin B, et al. Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes Care. 2012;35(5):1133–1138.
- Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Mol Cell Endocrinol. 2015;410:52–63. doi:10.1016/j.mce.2015.03.016.
(Visited 484 times, 1 visits today)







