Manuscript accepted on :23-May-2019
Published online on: 24-07-2019
Plagiarism Check: Yes
Reviewed by: Hind Shakir
Second Review by: M. Mohan Varma
Final Approval by: Prof. Juei-Tang Cheng
1Directorate General of Drug Administration, Mohakhali, Dhaka, Bangladesh.
2Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh.
Corresponding Author E-mail: amranms@du.ac.bd
DOI : https://dx.doi.org/10.13005/bpj/1733
Abstract
The monitoring of drug safety is a crucial element for the effective use of medicines to maintain high-quality medical care. The aim of this study was to analyze the adverse drug reactions (ADRs) as well as improve drug safety through pharmacovigilance (PV) in Bangladesh. The research work was conducted on the basis of a questionnaire by taking interviews of targeted stakeholders including academicians, doctors, pharmacists, manufacturers of drugs and directorate general of drug administration (DGDA) personnel. The study was conducted on 496 participants at Dhaka Metropolitan Dhaka, Rajshahi and Khulna Divisions from July 2015 to June 2018. Outcomes showed that among the interviewed populations 23% were female and 77% were male. Among participants, 66.9% of the interviewee was postgraduate degree holders. 62.7% respondents were familiar with the word element PV and 37.3% were ignorant of it. The major problem of DGDA to spreading the knowledge of PV was less manpower (73.9%). Among the factors that must be stopped to avoid the ADRs were the unethical practice of the healthcare professionals (50%). The topmost prioritized component was an education for knowledge (71.4%). It was found that many of the facilities for adverse drug reaction monitoring (ADRM) were absent in Bangladesh. The manpower and strength of DGDA must be increased to perform adequate monitoring and control as per the need of the country. We recapitulate that more research and development programs on PV activities in the country to improve the quality healthcare services is needed.
Keywords
Adverse Drug Reactions; Directorate General of Drug Administration; Drug Safety; Pharmacovigilance; Self-Medication
Download this article as:| Copy the following to cite this article: Hossain A. M, Amran S. M. A Cross-Sectional Pilot Study on Pharmacovigilance to Improve the Drug Safety in Bangladesh. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Hossain A. M, Amran S. M. A Cross-Sectional Pilot Study on Pharmacovigilance to Improve the Drug Safety in Bangladesh. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2Y1dGl7 |
Introduction
Pharmacovigilance (PV) is the study of detecting the undetected adverse effects of any drug after global marketing when the drug is consumed by millions of patients over the years.1 PV data are vital to ensure on-going safety and effectiveness of medicines and to provide information concerning regulatory actions such as drug safety alerts, labeling changes to the product information, drug recalls or withdrawal of a drug from the market.2 These activities are important for identifying adverse events and understanding, to the extent possible, their nature, frequency, and potential risk factors.3 Education and training of healthcare professionals in medicine safety, exchange of information between national PV centers, the coordination of such exchange and the linking of clinical experience of medicine safety with research and health policy, all serve to enhance effective patient care.
National PV and ADR reporting systems in India, Uganda, and South Africa are in their infancies and are not yet functioning optimally.4 This is due to lack of human, technical and financial resources.4,5 The lack of staff trained in PV seems to be the most serious limitation for the development of PV in low and middle-income countries.5 According to the WHO, in many developing countries patients are not adequately safeguarded from accessing harmful and ineffective medicines due to poor PV systems.6 This may result in treatment failures. Particular attention needs to be paid to proper infrastructure and governance, adequate human resources, training and capacity-building and sustainable methodologies and innovation in PV.5
The WHO Uppsala monitoring program recommends that, ideally, a national PV center should send over 200 reports per million inhabitants per year.4,7 The reporting rate for ADRs was low. India, with a population of approximately 1.24 billion, had a rate of PV reporting below 1%.8 This low rate was attributed mostly to the lack of training of physicians and pharmacists, and to a poor initiative in reporting ADRs.9 It is impossible to identify all safety concerns during controlled clinical trials. Once a product is marketed, there is generally a large increase in the number of patients exposed to a particular drug. Therefore, post-marketing safety data collection and clinical risk assessment based on observational data by physicians, pharmacists, and nurses are critical for evaluating and characterizing a product’s risk profile and for making informed decisions on risk minimization.10
PV has been started in Bangladesh at DGDA in 1996 with the support of WHO. Then ADRM Cell was established and some steps were taken to build awareness and communication. But the initiatives become dormant owing to lack of adequate support, funding, legislation, knowledge, and attitudes of the stakeholders. However, in order to improve ADR detection, these activities need to be promoted. Currently, there is no specific data about ADR in Bangladesh. Therefore, the main objectives of the current work were to find out the ways of avoiding ADRs in Bangladesh and to prioritize the potential components of PV needs of the country to make it sustainable as well as to identify the problems of DGDA in Bangladesh in handling the issues of PV.
Materials and Method
Study Area and Duration
The study was conducted at Dhaka Metropolitan, Dhaka, Rajshahi and Khulna Divisions from July 2015 to June 2018. The prevalence of pharmaceutical industries and medical college and hospitals are higher in the selected areas. During the study period, a total of 496 participants were selected.
Study Population and Experimental Design
In this cross-sessional study, out of total 600, 496 participants were interested to participate. The random sampling method was used for the study. From Dhaka division and metropolitan area 12 medical colleges, 15 hospitals were selected. From Rajshahi division 4 medical colleges, 8 hospitals were selected. From Khulna division, 5 medical colleges and 8 hospitals were selected for this study. From 3 divisions 78 medicine manufacturing industries were selected. DGDA of Bangladesh was selected as a single most important unit. Sample distribution of the study was shown in Table 1.
Table 1: Summary of the respondents by different types and areas.
| Respondents | Dhaka Metro | Dhaka Division | Rajshahi Division | Khulna Division | Total |
| Doctor | 40 | 30 | 29 | 35 | 134 |
| Pharmacist | 38 | 41 | 31 | 30 | 140 |
| Academician | 27 | 21 | 29 | 26 | 103 |
| Medicine Manufacturer | 15 | 25 | 21 | 17 | 78 |
| DGDA Inspector | 17 | 10 | 7 | 7 | 41 |
| Total | 138 | 126 | 117 | 115 | 496 |
Data Collection
Data were collected from the capital and the field level conducting an interview, discussion and observation. A self-made questionnaire (see Supplementary Material) was used to collect data related to ADRs, drug safety and study related info.
Ethical Considerations
The study protocol was approved by the Ethics Committee of the Department of Pharmaceutical Chemistry, University of Dhaka, Dhaka, Bangladesh. This study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Statistical Analysis
Results were collected, interpreted and presented as mean and percentages. For statistical and graphical evaluations Microsoft Excel 2010 (Roselle, IL, USA) was used.
Results
Gender Variation of the Respondents
The research work was conducted by surveying on different stakeholders on the basis of a standardized questionnaire. The variation in the gender of the total stakeholders is shown in Figure 1 which showed that 77% of the respondents were males and remaining were females.
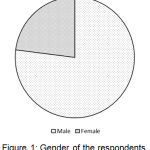 |
Figure 1: Gender of the respondents.
|
Educational Qualification of the Respondents
Figure 2 showed the qualification of the respondents. It was observed that the lowest 3.9% of the respondents were undergraduate and the highest number (66.9%) of the respondents was highly educated.
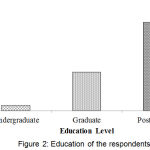 |
Figure 2: Education of the respondents.
|
Respondent Category
Doctors, pharmacist, academician, manufacturer, and DGDA inspector personnel took part in the interview process. The segmentation of respondent category was shown in Figure 3. Highest 28.2% participants were pharmacists and lowest 8.3% participants were DGDA inspector. Figure 4, represents the distribution of response by division. Knowledge and awareness of the participants related to PV are presented in Table 2. Among the respondents, DGDA inspector was maximum (Table 3) in terms of knowledge and idea. Similarly, DGDA inspector (Table 4) had more knowledge and ideas regarding PV in comparison to other respondents.
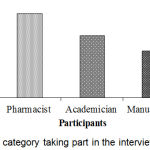 |
Figure 3: Respondent category taking part in the interview process.
|
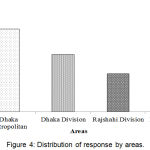 |
Figure 4: Distribution of response by areas.
|
Table 2: Knowledge and idea on pharmacovigilance and related terms.
| Indicators | Known (n, %) | Unknown (n, %) |
| How much familiarity of the word ‘Pharmacovigilance’? | 311 (62.7 %) | 185 (37.3 %) |
| How to write shortly the word ‘Pharmacovigilance’? | 311 (62.7 %) | 185 (37.3 %) |
| It is mainly needed for what? | 418 (84.3 %) | 78 (15.7 %) |
| Which phase of Clinical Trial does the ‘Pharmacovigilance’ is? | 170 (34.3 %) | 326 (65.7 %) |
| What does ‘ADR’ mean? | 423 (85.3 %) | 73 (14.7 %) |
| Either the respondent found or not the ADR reporting form | 288 (58.1 %) | 208 (41.9 %) |
| How many main parts are there in the ADR reporting form? | 156 (31.5 %) | 340 (68.5 %) |
| Who is the Chairman of ADR advisory committee? | 282 (56.8 %) | 214 (43.2 %) |
Table 3: Average number of response by the characteristics of respondents. Total number of indicator considered eight (8).
| Characteristics | Category | Average number of indicator answered* |
| Gender of the respondent | Male | 6.14 |
| Female | 5.82 | |
| Education of the respondent | Undergraduate | 4.26 |
| Bachelor | 5.85 | |
| Masters & above | 6.30 | |
| Type of respondent | Doctor | 6.00 |
| Pharmacist | 6.49 | |
| Academician | 5.54 | |
| Manufacturer | 5.51 | |
| DGDA Inspector | 7.24 | |
| Division/Area | Dhaka Metropolitan | 6.62 |
| Dhaka Division | 6.29 | |
| Rajshahi Division | 6.12 | |
| Khulna Division | 5.11 |
Table 4: Indicator shows the level of knowledge and idea of the respondents.
| Characteristics | Category | Average score in 8 indicators/aspects considered* |
| Gender | Male | 0.60 |
| Female | 0.57 | |
| Education | Undergraduate | 0.36 |
| Bachelor | 0.53 | |
| Masters & above | 0.64 | |
| Type of respondent | Doctor | 0.58 |
| Pharmacist | 0.65 | |
| Academician | 0.54 | |
| Manufacturer | 0.49 | |
| DGDA Inspector | 0.81 | |
| Area/ Division | Dhaka Metropolitan | 0.70 |
| Dhaka Division | 0.62 | |
| Rajshahi Division | 0.57 | |
| Khulna Division | 0.46 | |
| Mean | 0.60 |
Note: * Score ‘1’ and ‘0’ has been assigned respectively for ‘yes’ and ‘no’ responses.
Problems of DGDA have shown in Figure 5. The result showed that 73.9% respondents replied that less manpower was the main problem of DGDA which was the maximum and minimum respondents (1.8%) replied that the lack of CHRD was the main problem of DGDA.
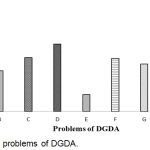 |
Figure 5: Main problems of DGDA.
|
Here, A: Less Manpower; B: Legislative weakness; C: Knowledge and Skill deficiency; D: Logistic Support; E: Physical facilities; F: Non- awareness of the beneficiaries; G: Lack of Laboratory Service; H: Excess number of Industry & outlets; I: Lack of CHRD.
Things must be stopped immediately to avoid ADRs have been shown in Figure 6. The result showed that the things we must stop to avoid ADRs. They are the unethical practice of the healthcare professionals (50%), distribution/purchase of medicine to/from any unauthorized source (46%), polypharmacy (44.2%), self-medication and Improper storage and distribution of drugs (40.7%).
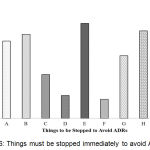 |
Figure 6: Things must be stopped immediately to avoid ADRs.
|
Here, A: Self-medication; B: Poly Pharmacy; C: Aggressive promotion & push sale of drug products; D: Counterfeit, Sub-standard and Less effective or expired drugs in the market; E: Unethical practice of the Healthcare providers; F: Irrational use of Antibiotics, Steroids and other drugs; G: Registration of more combination products if alternative options are there; H: Distribution/ Purchase of medicine to/ from any unauthorized source; I: Improper storage and Distribution of drugs.
Components of the PV require immediate action for sustainable PV has shown in Figure 7. The result shows that the topmost prioritized component was an education for knowledge (71.4%).
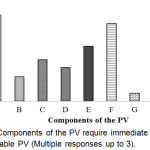 |
Figure 7: Components of the PV require immediate action for sustainable PV (Multiple responses up to 3).
|
Here, A: Education for knowledge; B: Training for skill; C: Legislation; D: Statutory/ Regulations; E: Recruitment of Graduate Pharmacists in all Hospitals & Pharmacies; F: Strengthening the Laboratory service; G: Strengthening the National PV Centre (ADRM cell); H: PV awareness program & effective communication; I: Introduction PV in the course curriculum for Medical, Pharmaceutical and Health educations.
Whether there were not to have graduate pharmacists in hospitals and pharmacies is a vital problem for conducting PV had shown in Figure 8. From the result, it was found that the highest percentage (53.2%) of the respondents strongly agreed that for conducting PV, it is a vital problem not to have graduate pharmacists in hospitals and pharmacies which was the maximum.
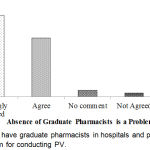 |
Figure 8: Not to have graduate pharmacists in hospitals and pharmacies is a vital problem for conducting PV.
|
Importance of PV for drug safety has shown in Figure 9. From the result, it was found that 72.8% respondents opined that PV was extremely important for drug safety which was the maximum.
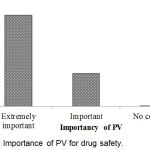 |
Figure 9: Importance of PV for drug safety.
|
Components needed to improve the attitude of the healthcare professionals and industry people have shown in Figure 10. From the result, it was found that all the components mentioned in the figure were 43.8% which was the highest one.
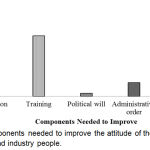 |
Figure 10: Components needed to improve the attitude of the healthcare professionals and industry people.
|
It was found that the major portion of the respondents (57.1%) didn’t write a few words in this regard (Figure 11). It may be due to the lacking or limited idea/knowledge regarding PV may also have other reasons.
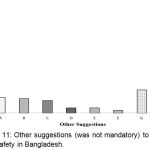 |
Figure 11: Other suggestions (was not mandatory) to improve drug safety in Bangladesh.
|
Here, A: To control/regulate strictly the pharmacies and village doctors; B: To develop public awareness on Adverse Effect of medicine and on reporting system through media; C: Not to sell medicine without prescription of regd. Qualified doctors and to ensure the Rational Use of Drugs; D: To develop qualified healthcare professionals (HCPs) through adequate training, education and more research programs; E: To conduct BE and CT; F: To ensure good quality RM for medicine manufacturing; G: Others; H: Not response.
Discussion
PV is a fruitful process for determining and making a response to the risk-benefit issues initiating from the freshly marketed medicine 11. In this study, we examined ADRs and improve drug safety through PV in Bangladesh. In our study, it can be observed that the percentage of respondents among the male (77%) was higher than female (23%).
Healthcare professionals (HCPs) play a pivotal role in the PV system. They need extensive knowledge and proficiency in the area of safe medication which will efficaciously lead to this field through initial recognition, management, and reporting of the safety medicine issues.12 Furthermore, increased participation of HCPs from private and public sectors, pharmaceutical industries, academic organizations as well as the public at large is required to ensure the safety medication. In this study, the majority of the respondents possessed educational qualifications, with 66.9% having gained a postgraduate degree and 3.9% educated to undergraduate level. Among them, the highest 28.2% of respondents were pharmacist while the lowest 8.3% of respondents were DGDA inspector.
The idea of PV has been broadly recognized globally. Furthermore, the knowledge and concept on PV and associated terms of HCPs greatly involve with prescription, choice of drugs, monitoring, and copious other health issues that comprises various concepts of certain medicinal care by way of assuring the innocuous and operative usage of pharmaceutical medications, ameliorating patient satisfaction as well as quality of lifestyle, enhancing economic benefits, and preventing medication errors and ADRs.13 From our study, it was found that 62.7% of respondents knew about the term PV and 37.3% of respondents did not know simply the word PV. In addition, 85.3% of respondents knew the meaning of ADR and 14.7% of respondents did not know. Allela, et al.13 showed that approximately 62.4% of respondents knew the term PV whereas, about 68.8% of respondents knew the minimum information required for the submission of an initial ADR report.
Moreover, in our study, the knowledge of PV and ADR reporting among HCPs was assessed by asking 8 questions with yes/no options. A score of 1 was given for each yes response and 0 for each no response. The mean score in 8 indicators answered for male and female respondents was 0.60 and 0.57 respectively. According to the study of Allela, et al.13 revealed that the mean knowledge score of PV and ADR reporting for male and female respondents was 5.77 and 5.4 successively. Our study obviously showed that some of the respondents do not comprehend the concept of PV. Therefore, instructive training programs play an essential role in enhancing the knowledge of HCPs concerning ADR reporting requirements.
Medication safety is an immense issue in the health care organization. In Bangladesh, the DGDA is playing a crucial role in assuring safe, fruitful and quality medicines to maintain the health of the people of the country. This study exposed that due to some problems of DGDA, they do not perform their responsibility efficiently. The result showed that 73.9%, 40.3% and 35.5% of respondents thought that less manpower, knowledge and skill deficiency and lack of laboratory service respectively was the main problem of DGDA. According to the study of Biswas et al.14 it was found that the government agency of Asian countries face several challenges in PV greatly such as lack of manpower and economic resources within the regulatory agencies, absence of PV specialists, lack of consciousness on PV between physicians and public that essential to be lessened, with the purpose of building a healthy system for the future.
Nowadays, the rate of morbidity and mortality is increased due to ADRs. Research showed that in the USA estimate for about 30% of hospital admissions,15 and cost around $170 billion yearly because of ADRs.16 Geriatric patients are at high risk of suffering ADRs, most of which can be preventable.15 There are different factors that are responsible for ADRs. In our study, we have observed that 50% and 44.2% of respondents thought that the unethical practice of the healthcare professionals and polypharmacy correspondingly was the main things for ADRs. Ahmed et al.17 revealed that the incidence of ADRs with polypharmacy was 10.5% among aged patients.
The components of PV are important for identifying, estimating, and mitigating adverse effects associated with drugs as well as other pharmaceutical medications for achieving sustainable PV system. In this study exhibited that 71.4% and 64.3% of respondents replied that the most essential components were an education for knowledge and strengthening the laboratory service. According to the study of Nahar et al.,18 revealed that the response of reporting of ADRs from teaching hospital was 55%.
The system of PV is very essential to keep up the safety information, identification and assessment of medication safety indications through the handbook and medical instruments reporting. In our study, it was found that 72.8% respondents opined that PV is extremely important for drug safety. Amedome et al.19 revealed that about 74% of respondent specified an identification of medication safety as the most significant purpose of PV.
Conclusions
In Bangladesh most of the facilities for ADRM are the absent and unethical practice of the healthcare professionals is the main causative factor for ADRs. The manpower and strength of DGDA must be increased to perform adequate monitoring and control as per need. All the healthcare professionals must be brought under regulations so that the system of medical practice and prescription become ethical. Collaborative actions between government and private institutions and moral obligations must be ensured. Education for knowledge regarding PV to be ensured in the post-graduation level of education for the healthcare professionals and also in other educations. In order to recruit the “Graduate Pharmacists” in all the hospitals and pharmacies, so that they can play their better role in drug safety and patient care. Furthermore, it’s obligatory to conduct more research and development programs on PV activities in the country as per the need to improve the quality of service.
Limitation
The present study was conducted in 4 research areas of Bangladesh with only 496 volunteers. It would be best if we could complete this study in numerous areas all over the country.
Conflict of Interest
There is no conflict of interest.
Acknowledgements
The authors are grateful to the following:
DGDA
American World University
Md. Sahab Uddin, and Abdullah Al Mamun, Department of Pharmacy, Southeast University, Dhaka, Bangladesh
Supplementary Material
See the attached supplementary material.
References
- WHO. WHO | Pharmacovigilance. WHO. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/. Published 2015. Accessed February 28, 2019.
- Bégaud B, Evreux JC, Jouglard J, Lagier G. [Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France]. Therapie. 1985;40(2):111-118. http://www.ncbi.nlm.nih.gov/pubmed/4002188. Accessed February 28, 2019.
- WHO. WHO | The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). WHO. https://www.who.int/classifications/atcddd/en/. Published 2010. Accessed February 28, 2019.
- Meyboom RHB, Egberts ACG, Edwards IR, Hekster YA, de Koning FHP, Gribnau FWJ. Principles of Signal Detection in Pharmacovigilance. Drug Saf. 1997;16(6):355-365. doi:10.2165/00002018-199716060-00002.
- Meyboom RHB, Gribnau FWJ, Hekster YA, de Koning GHP, Egberts ACG. Characteristics of Topics in Pharmacovigilance in The Netherlands†. Clin Drug Investig. 1996;12(4):207-219. doi:10.2165/00044011-199612040-00006.
- MDB S. The Detection of New Adverse Drug Reactions – Google Books. 2nd ed. Stocklons Press, New York; 1998. https://books.google.com.bd/books?id=IjVdDwAAQBAJ&pg=PR5&lpg=PR5&dq=Stephens+MDB,+1988&source=bl&ots=GM23j4DJ3D&sig=ACfU3U0ig8GL1WvhheMXGTgYZpXoM4alcg&hl=en&sa=X&ved=2ahUKEwiavaWX497gAhXTXisKHYeJC48Q6AEwAnoECAoQAQ#v=onepage&q=Stephens MDB%2C 1988&f=fa. Accessed February 28, 2019.
- WHO. WHO | WHO Collaborating Centre for International Drug Monitoring. WHO. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/collab-centre-uppsala/en/. Published 2015. Accessed February 28, 2019.
- Venulet J. Aspects of standardization as applied to the assessment of drug-event associations. Drug Inf J. 1984;18(3-4):199-210. http://www.ncbi.nlm.nih.gov/pubmed/10268551. Accessed February 28, 2019.
- Royer RJ, Benichou C. [International reporting of adverse drug reactions. Final report of CIOMS ADR Working Group]. Therapie. 1990;46(3):173-178. http://www.ncbi.nlm.nih.gov/pubmed/1792648. Accessed February 28, 2019.
- WHO. Minimum Requirements for a Functional Pharmacovigilance System.; 2010. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/PV_Minimum_Requirements_presentation.ppt. Accessed February 28, 2019.
- Waller PC, Coulson RA, Wood SM. Regulatory Pharmacovigilance in theUnitedKingdom: Current Principles and Practice. Pharmacoepidemiol Drug Saf. 1996;5(6):363-375. doi:10.1002/(SICI)1099-1557(199611)5:6<363::AID-PDS249>3.0.CO;2-7.
- S N. Importance of Pharmacovigilance and the Role of Healthcare Professionals. J Pharmacovigil. 2018;06(01):1-2. doi:10.4172/2329-6887.1000252.
- B. Allela OQ, Elkalmi RM, Salih AS, Yousif DY, Ali HW, Shammo NO. Pharmacovigilance Knowledge and Perceptions Among Pharmacy, Medical and Nurse Students in University of Duhok. J Pharm Pract Community Med. 2018;4(2):60-65. doi:10.5530/jppcm.2018.2.16.
- Biswas P. Pharmacovigilance in Asia. J Pharmacol Pharmacother. 2013;4(Suppl 1):S7-S19. doi:10.4103/0976-500X.120941.
- Leporini C, De Sarro G, Russo E. Adherence to therapy and adverse drug reactions: is there a link? Expert Opin Drug Saf. 2014;13(sup1):41-55. doi:10.1517/14740338.2014.947260.
- Heller MK, Chapman SCE, Horne R. Beliefs about medication predict the misattribution of a common symptom as a medication side effect — Evidence from an analogue online study. J Psychosom Res. 2015;79(6):519-529. doi:10.1016/j.jpsychores.2015.10.003.
- Ahmed B, Nanji K, Mujeeb R, Patel MJ. Effects of polypharmacy on adverse drug reactions among geriatric outpatients at a tertiary care hospital in Karachi: a prospective cohort study. PLoS One. 2014;9(11):e112133. doi:10.1371/journal.pone.0112133.
- Nahar N, Karim A, Paul P, Khan T. Response of Reporting Adverse Drug Reactions among Medical Practitioners. Bangladesh Med J. 2011;40(2):13-18. doi:10.3329/bmj.v40i2.18498
- SN A, BA D. Pharmacovigilance Practices: Knowledge and Attitudes among the Healthcare Professionals at the Volta Regional Hospital of Ghana. J Pharmacovigil. 2017;05(03):1-5. doi:10.4172/2329-6887.1000229.








