Manuscript accepted on :11-April-2019
Published online on: 09-05-2019
Plagiarism Check: Yes
Reviewed by: Felix-Martin Werner
Second Review by: Mr. Guy-Armel Bounda
Miss Pamila, Ramya Sugumar* and Darling Chellathai David
Department of Pharmacology SRMC, SRIHER, Porur, Chennai, Tamil Nadu 600116, India.
Corresponding Author E-mail: drramya.sugumar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1689
Abstract
In this study we evaluated the possible beneficial drug- interaction between Roflumilast (BCRP inhibitor) and Methotrexate (BCRP substrate) on viability of primary squamous cell carcinoma cell line using an in vitro technique. The KB cell line was treated with Roflumilast and Methotrexate to evaluate its anticancer activity using MTT assay. Image analysis under phase contrast microscopy was performed and flow-cytometry was done to see for cell cycle arrest as a result of drug treatment. Cell viability gradually decreased with the increasing concentrations of roflumilast, methotrexate and the cytotoxic effect with the combination of roflumilast and methotrexate also increased proportionally. Phase contrast microscopy indicated characteristic features of apoptosis which was confirmed in flow cytomtery and indicated cell cycle arrest in M phase. Efflux pump mediated multidrug resistance being a common feature among all cancers, the results of our study evidence the use of combined methotrexate and roflumilast to overcome drug resistance by exploiting the fact that the former is a BCRP substrate and latter a BCRP inhibitor. By combining the two drugs, it allows optimization of therapy by dose reduction of methotrexate and roflumilast and thereby resulting in better efficacy.
Keywords
Anti Cancer; BCRP Inhibitor; BCRP Substrate; Methotrexate; Roflumilast
Download this article as:| Copy the following to cite this article: Pamila M, Sugumar R, David D. C. Evaluation of Roflumilast (BCRP Inhibitor) and Methotrexate (BCRP Substrate) on Viability of Primary Squamous Cell Carcinoma – An In Vitro Study. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Pamila M, Sugumar R, David D. C. Evaluation of Roflumilast (BCRP Inhibitor) and Methotrexate (BCRP Substrate) on Viability of Primary Squamous Cell Carcinoma – An In Vitro Study. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2Jb72kE |
Introduction
The up regulation of breast cancer resistance protein (BCRP), an ATP-binding cassette (ABC) transporter causes active efflux of anticancer drugs thereby making the cancer cells resistant to them. BCRP is identified as a molecular basis of multidrug resistance (MDR) in varied cancer cells. BCRP, a transmembrane protein is involved in transport of substrates out of cells utilizing ATP. It is implicated in multi drug resistance (MDR) of several cancers where it is over expressed and pumps out the cytotoxic drugs resulting in development of resistance.1 This protein identifies substrates from various anticancer drugs of conventional chemotherapeutics and also those which are relatively new in use. Therefore, it can be a therapeutic target for cancer treatment where one can block the BCRP-mediated active efflux thereby enhancing the drug efficacy. However these biologically active molecules may have a complicated pathway via which they act, thereby defining precise mechanisms of BCRP gene expression may identify a novel molecular target to modulate BCRP-mediated resistant. Untangling the mechanism would develop a way to modulate BCRP function to aid effective cancer treatment.
In primary squamous cell carcinoma the expression of BCRP is increased by several folds causing loss of efficacy of drugs against cancer like methotrexate, which is a BCRP substrate.3,4 Therefore development of drugs which inhibit the function of BCRP serve valuable in increasing the bioavailability of anticancer drugs which are BCRP substrates and may overcome the emergence of MDR cancers.2 Very recently an in-silico study demonstrated roflumilast to possess potential BCRP inhibitory property.5 Therefore, in this study we evaluated the possible beneficial drug- interaction between Roflumilast (BCRP inhibitor) and Methotrexate (BCRP substrate) on viability of primary squamous cell carcinoma cell line using an in vitro technique.
The main objective of our study was to evaluate the combined anticancer effect of methotrexate and roflumilast and compare it with that of methotrexate alone on primary squamous cell carcinoma cell line to understand the anticipated favourable drug-interaction.
Methodology
Cell line and Reagents
The KB cell line (ubiquitous KERATIN-forming tumor cell line) was obtained from National Center Cell Science, Pune, India. According to instruction provided in earlier reports, cell line was maintained in Minimal Essential Medium (MEMS), supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) and was kept in a humidified atmosphere of 50 μg/ml CO2 at 37°C.
Evaluation of Anti-Cancer Activity was Done Using In Vitro MTT Assay
For evaluation of anticancer activity, the cells were placed in 96 well plates and kept in incubation with 5% CO2 at 37°C. Once the cell reached the confluence, various concentrations of methotrexate and roflumilast in combination and alone were added into well followed by a 24-hour incubation period. After incubation for prescribed time periods, the images were captured using phase contrast microscopy (Nikon, Japan). During successive follow up, cells which were attached with well were removed by trypsanization and washed with phosphate buffered saline (pH 7.4) without serum. MTT (100µl/well; 5mg/ml) was added to each well and incubated for next 4 hours. At prescribed time 300 µl/well of DMSO was added in all wells to solubilise the precipitates. The absorbance of each well and a blank was measured at 595 nm using a UV spectrophotometer. Experiment was repeated three times and the average was considered for the final conclusion. IC50 concentrations required for 50% inhibition of cell viability was determined graphically and % cell viability was calculated as follows:
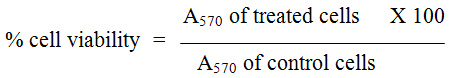
Flow-Cytometry for Cell Cycle Distribution
Cell line was grown and treated with IC50 values of compounds as per standard procedures for 24 hours. After 24 hours, cells were trypsinized and collected by centrifugation at 3000 rpm for 10 minutes. The cells were fixed using 70% alcohol at 20°C overnight. According to manufactures’ instruction cell cycle reagent was added, incubated in dark for 30 minutes and was analyzed using a flow cytometer (Millipore, Guava).
Results
The in vitro cytotoxic effects of various concentrations of roflumilast, methotrexate and its combination were tested on KB cell lines and the results of which are depicted in Table 1. Cell viability was seen to gradually decrease with the increasing concentrations of roflumilast, methotrexate and the cytotoxic effect of the combination of roflumilast and methotrexate also increased proportionally. Methotrexate was more effective in inhibiting the cell viability in comparison to roflumilast independently. While, the roflumilast and methotrexate combination produced more significant inhibition in cancer cell survival. The maximum inhibition in % cell viability was observed at 1000µg/ml with methotrexate, roflumilast and its combination. The IC50 values of roflumilast, methotrexate and combination of roflumilast and methotrexate were 125 µg/mL, 62.5µg/mL and 31.2µg/mL respectively (Table 1 & Figure 1).
Table 1: In vitro cytotoxicity assay of test drug against KB Cell lines.
| Sample | |||
| Concentration (µg/mL) | % Inhibition of Cell viability MTT Assay | ||
| Concentration (µg/mL) | Roflumilast | Methotrexate | Roflumilast + Methotrexate |
| 0 | 100 | 100 | 100 |
| 7.8 | 17.65 | 29.17 | 33.8 |
| 15.6 | 25.41 | 36.43 | 41.68 |
| 31.2 | 33.67 | 43.68 | 48.94 |
| 62.5 | 41.43 | 50.07 | 55.57 |
| 125 | 49.82 | 57.83 | 61.21 |
| 250 | 57.2 | 64.96 | 67.59 |
| 500 | 65.34 | 71.72 | 72.72 |
| 1000 | 73.47 | 78.98 | 80.48 |
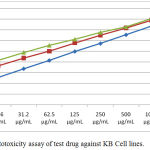 |
Figure 1: In vitro cytotoxicity assay of test drug against KB Cell lines.
|
Phase contrast microscopy analysis showed that (Figure 2) after the treatment with drug alone or in combination, cells were detached from surface and showed bubbling and shrinking appearance indicating that cells have moved to apoptosis. Further the combined cytotoxic effect of roflumilast and methotrexate was evaluated using flow cytomtery and the maximum inhibition of cell viability was observed at M phase of cell cycle with a 67.76% cell arrest (Figure 3).
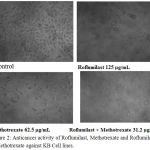 |
Figure 2: Anticancer activity of Roflumilast, Methotrexate and Roflumilast + Methotrexate against KB Cell lines. |
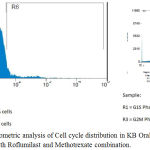 |
Figure 3: Flow Cytometric analysis of Cell cycle distribution in KB Oral Cancer cell lines treated with Roflumilast and Methotrexate combination.
|
Discussion
Among the several mechanisms contributing to cancer chemotherapeutic drug resistance, efflux pumps mediated resistance is gaining immense importance. Breast cancer resistance protein (BCRP) is one such transmembrane protein implicated in multi drug resistance (MDR) of several cancers due to its overexpression and extrusion of cytotoxic drugs resulting in development of resistance.1 It is well known that BCRP is expressed in high folds in primary squamous cell cancer.1 In the present study the possible beneficial drug- interaction between Roflumilast (BCRP inhibitor) and Methotrexate (BCRP substrate) on viability of primary squamous cell carcinoma cell line using an in vitro technique was studied.
In our study a dose dependent increase in inhibition of % cell viability was observed with roflumilast and methotrexate as separate single treatment with IC50 at 125µg/L and 62.5µg/L of roflumilast and methotrexate respectively (Table 1). Methotrexate is a standard anticancer drug used in clinical practice while roflumilast which is a drug used for COPD, in this study has shown to possess significant anticancer activity. This anticancer activity of roflumilast may be attributed to its phosphodiesterase inhibitory mechanism contributing to high intracellular levels of cAMP which can effectively kill cancer cells in vitro.8
When roflumilast and methotrexate were combined and analysed for their cytotoxic effect it was noticed that at all concentrations (7.8 – 1000µg/L) there was a marked and significant inhibition of % cell viability when compared to each single treatment. This is in alignment with our hypothesis of anticipated favourable drug interaction between the two drugs. Roflumilast being a BCRP inhibitor has prevented efflux of methotrexate (BCRP substrate) and increased the bioavailability of methotrexate. The above results of favourable drug interaction were confirmed by Flow Cytometric analysis of Cell cycle distribution in KB Oral Cancer cell lines treated with Roflumilast and Methotrexate combination which showed maximum cell viability inhibition in M phase of cell cycle. However, a flowcytometry study of methotrexate on astrocytes in primary culture had shown a cell cycle arrest in M phase.9
Recently a study conducted by Montanari et all demonstrated Roflumilast to possess BCRP inhibitory activity by in silico virtual screening methods.5 Whereas several studies have shown methotrexate to be a BCRP substrate. Keeping this in the background, this is a first of its kind preliminary in vitro study to explore the drug interaction between a BCRP inhibitor (Roflumilast) and BRCP substrate (Methotrexate) which has emerged worthwhile.
Conclusion
Multidrug resistance due to overexpression of efflux pumps is an important cause of therapeutic failure in cancer chemotherapy. BCRP is known to have increased expression in primary squamous cell cancer and methotrexate is one of the standard regimen drugs used in the management of the same. In this study we wanted to evaluate the cytotoxic effect of combined methotrexate which is a known substrate for BCRP and roflumilast whose BCRP inhibitory activity is postulated by virtual screening studies. We obtained results evidencing favourable increase in cytotoxic effects on KB cell line of the combination when compared to single treatment.
Summary
Efflux pump mediated multidrug resistance being a common feature among all cancers, the results of our study evidence the use of combined methotrexate and roflumilast to overcome drug resistance by exploiting the fact that the former is a BCRP substrate and latter a BCRP inhibitor. By combining the two drugs, it allows optimization of therapy by dose reduction of methotrexate and roflumilast and thereby resulting in better efficacy. This may pave a path for novel anticancer therapy after further mechanistic in vitro and in vivo studies for confirmatory elucidation of roflumilast BCRP inhibitory activity.
References
- Maliepaard M, Scheffer GL, Faneyte IF. Subcellular Localization and Distribution of the Breast Cancer Resistance Protein Transporter in Normal Human Tissues. Cancer Res. 2001;61: 3458–3464.
- Breedveld P, Beijnen JH, Schellens JHM. Use of P-Glycoprotein and BCRP Inhibitors to Improve Oral Bioavailability and CNS Penetration of Anticancer Drugs. Trends Pharmacol. Sci. 2006;27: 17–24.
- Friedrich RE, Punke C, Reymann A. Expression of multi-drug resistance genes (mdr1, mrp1, bcrp) in primary oral squamous cell carcinoma. In vivo. 2004;18(2):133-47.
- Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7: E118–E133.
- Montanari F, Cseke A, Wlcek K, Ecker GF. Virtual screening of drug bank reveals two drugs as new BCRP inhibitors. J Biomol Screen. 2017; 22(1): 86–93.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 2016; 65(1-2):55-63.
- Dhanya NV, Selvi S. Anticancer activity and cell cycle arrest evoked ethanolic extract of Cyperus Rotundus in KB oral cancer cell lines. International Journal of Pharma and Biosciences. 2017; 8(2): 757-765.
- Hirsh L, Dantes A, Suh BS, Yoshida Y, Hosokawa K, Tajima K et al. Phosphodiesterase inhibitors as anti-cancer drugs. Biochem Pharmacol. 2004 Sep 15;68(6):981-8.
- Bruce-Gregorios JH, Soucy D, Chen MG, Benson N. Effect of methotrexate on cell cycle and DNA synthesis of astrocytes in primary culture: flow cytometric studies. J Neuropathol Exp Neurol. 1991;50(1):63-72.







