Manuscript accepted on :04-June-2019
Published online on: 25-06-2019
Plagiarism Check: Yes
Reviewed by: Jobin Christ
Second Review by: Khalil Alhadidy
Enas R. Abdel Hamid1 , Shaimaa A. Hashem*1
, Shaimaa A. Hashem*1 , Lobna S. Sherif1
, Lobna S. Sherif1 , Hanaa H. Ahmed2
, Hanaa H. Ahmed2 , Amal I. Hassanain1
, Amal I. Hassanain1 , Amira Ahmed3 and Nayera E. Hassan4
, Amira Ahmed3 and Nayera E. Hassan4
1Child Health Department, National Research Centre, Dokki, Giza, Egypt.
2Hormones Department, National Research Centre, Dokki, Giza, Egypt.
3El-Galaa Teaching Hospital, Cairo, Egypt.
4Biological Anthropometry Department, National Research Centre, Dokki, Giza, Egypt.
Corresponding Author E-mail: Sho_hashem@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1669
Abstract
Mercury is a ubiquitous cumulative element which is existed and contaminated in many forms; the most common route is fish intake. The purpose of this work was to study the link between the rate of fishy food consumption and serum mercury level in both pregnant mothers and cord blood of their newborns. This study consisted of 70 pregnant women and their neonates. The study samples were collected from the maternal blood and cord blood of their newborns at the delivery time. The mean of gestational age for their neonates is (36.9 ± 2.1) weeks. Detailed food frequency questionnaire was taken to evaluate the frequency of fish consumption per week, and approximate frequency per month. Measurement of serum mercury level was done using inductive coupled plasma mass spectrometry. This study finds highly significant increase (P ˂0.01) in serum mercury level either in maternal blood or in their newborns’ cord blood in a manner dependent on the frequency of fish consumption. High significant positive correlation (P ˂0.01) has been recorded between maternal mercury level and cord blood mercury level of their newborns. Also, the frequency of fish consumption is high significantly correlated with mercury level in each of maternal blood and cord blood of their newborns (P ˂0.01). Hence, the study concludes that mercury tends to accumulate in fish food chains reaching the human beings. Frequency of fish consumption per month affects the level of mercury in mothers and their neonates.
Keywords
Fish Intake; Mercury; Newborns; Pregnant Mother
Download this article as:| Copy the following to cite this article: Hamid E. R. A, Hashem S. A, Sherif L. S, Ahmed H. H, Hassanain A. I, Ahmed A, Hassan N. E. Effect of Fish Frequency Consumption on Serum Mercury Levels in Pregnant Mothers and Their Newborns. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Hamid E. R. A, Hashem S. A, Sherif L. S, Ahmed H. H, Hassanain A. I, Ahmed A, Hassan N. E. Effect of Fish Frequency Consumption on Serum Mercury Levels in Pregnant Mothers and Their Newborns. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2NdV0tv |
Introduction
Mercury is a cumulative harmful toxin; it is ever-present in the environment, earth and livings that are totally unable to avoid exposure to some form of mercury [1]. Mercury is existed and contaminated in many forms; elemental mercury, organic mercury, and inorganic mercury. Humans can be exposed to mercury by different routes; ingestion, dermal contacts or inhalation [2].
Elemental mercury is used in such in thermometers, sphygmomanometers, and dental amalgam. It is badly absorbed unless vaporized form. Inorganic mercury is used in beauty products corrosive sublimates, pesticides, explosives, and as a preservative in some medicinal preparations like vaccines. Inorganic mercury itself is not feasible by ordinary routes. However, microorganisms can alter inorganic mercury to organic mercury, which is toxic. Organic mercury is a lipophilic substance that is predominantly in the form of ethyl or methyl mercury. Organic mercury is dispersed in all tissues due to its high permeability power crossing the blood-brain barrier and placenta [2-3].
A constellation of penetrating urbanization practices, technology, industry and metals processing are strongly associated with environmental pollution, human dietary practicing, and personal change in lifestyle [2]. Mercury comes into water by the wastes of industrial pollution. Methylmercury is the predominant form of mercury in fish and other seafood; it is methylated by algae and bacteria in water. The methylmercury has slow elimination rate and this leads to bio-accumulation as small fish is being eaten by bigger fish, so its toxicity increased by moving up in the food chain [4].
Methylmercury is lipid soluble compound that crosses the tissues easily, it crosses the blood brain barrier tending to accumulate in the central and peripheral nervous tissues, glomerular blood barrier affecting the kidneys, maternal-fetal barrier of the placenta reaching to the fetus. There is great variation in the content, type and style of pregnant women on fish consumption between countries. This is to some extent due to local environmental conditions, species availability and consumption preferences [1]. It is believed that maternal fish intake during pregnancy can affect the developing foetus soon and at the long run causing intrauterine growth restriction, low birth weight, premature labour, weight gain and neurodevelopmental growth [5-6].
The goal of the present investigation was to elucidate the relation between the rate of fishy food consumption and serum mercury level in both pregnant mothers and cord blood of their newborns in order to recommend balanced maternal fish consumption.
Materials and Method
This is a cross-sectional study randomly enrolled 70 pregnant mothers and their newborns from those attending El-Galaa Teaching Hospital for Obstetrics and Gynecology, Cairo, from September 2016 till June 2017. This study is a part of the in-house project that was approved by the Medical Ethical Committee of the National Research Center (16/295). All participants were informed about the objectives of the study and volunteered to participate with providing a written informed consent.
All Participants Were Subjected to the Following
Full history taking; stress on gestational and obstetric history.
Mothers with chronic illness, high risk pregnancies, and neonates with any genetic or congenital abnormalities were excluded from the study.
Food frequency questionnaire (FFQ); emphasis on fish consumption particularly; how many times per week, and approximate frequency per month. Then according to the reported frequency, the subjects were classified into three groups; none or once / month, 2-3 times/ month and more than 4 times / month.
Assessment of mother anthropometric measurements; the weights (kgs) of pregnant mothers were measured and total gestational weight gain was estimated by subtracting the early first trimester weight from the last measured weight before delivery (corrected to the nearest 0.1 gm), height (cm) (corrected to the nearest 0.1 cm), and body mass index [weight (kg)/height2 (meter)] according to WHO standards [7].
Neonatal examination and anthropometric measurements; full detailed physical examination was done. The anthropometric measurements of weight (kgs) (corrected to the nearest 0.1 gm), length (cm) (corrected to the nearest 0.1 cm), head circumference (cm) (corrected to the nearest 0.1 cm), and mid-upper arm circumference (cm) (corrected to the nearest 0.1 cm) were carried out [8], Apgar scoring was recorded at one and five minutes [9].
Laboratory Investigations
Five ml of maternal venous blood sample was taken by highly trained nurse at the early labour phase in normal vaginal delivery or at the preparation time for caesarian section, and kept in tube without EDTA. Ten ml of cord blood was taken at time of delivery before placental separation and put in EDTA free tubes. Both blood samples were then centrifuged at 1800 x g under cooling (4°C) to separate serum samples which were kept at -70 until analysis.
Measurement of mercury level in serum was done using inductive coupled plasma mass spectrometry (ICP-MS). It is obtained from Sigma- Aldrich, Australia, and labelled as Fluka Trace Cert Ultra. The hydrochloric acid and ultrapure nitric acid (HNO3) were taken from J.T. Baker Inc. The other solvents and reagents used as analytical grade were obtained from Sigma- Aldrich, Australia. Deionized water was used for washing the laboratory apparatus and glassware together with the standard solutions and sample preparation (resistance < 18 m, Academic Milli-Q Ultra-Pure Water System, Australia)[10].
Before starting the analysis, the ICP-MS instrument was optimized to give the highest possible signal intensities. Then the appropriate calibration standards were measured through using standard aqueous solutions diluted in the range of 0.05 to 10 mg/L. According to the protocol of quality assurance, at least six-point calibrations of different ranges for mercury (0.10 to 10 mg/L and 0.05 to 2000 μg/L) were done. From the corresponding calibration curve, the concentrations of mercury in the sample solution were determined.
Statistical Analysis
Data were collected, verified, coded and analyzed using the Statistical Package for Social Science (SPSS) version 23 (SSPS Inc., Pennsylvania, USA). Qualitative data presented as number and percentages. The comparison between two groups with qualitative data was done by using Chi-square test. Independent t-test was used to compare between two groups regarding quantitative data respectively. Pearson correlation analysis was used to assess the relation between two quantitative parameters in the same group. The p-value was considered significant at P <0.05.
Results
This cross section study involved 70 pregnant mothers and their newborns of both sexes. Table (1) shows the clinic-descriptive data studied for pregnant mothers and their newborns. Mother ages are ranged between 18- 40 years with (mean ±SD) 26.7 ± 5.6 years, their mean ±SD of gestational age is 36.9± 2.1 and the mean ±SD of neonatal birth weight is 2.9 ±0.6 Kg.
Table 1: Maternal and neonatal clinic-descriptive data.
| Clinic-descriptive data | Mean | ±SD |
| Maternal factors | ||
| Age of mother | 26.7 | ± 5.6 |
| Weight | 75.4 | ± 14.83 |
| Height | 157.7 | ± 6.668 |
| neonatal factors | ||
| Birth weight | 2.9 | ± 0.6 |
| Birth length | 47.4 | ± 3.3 |
| HC | 34.2 | ± 1.9 |
| MAC | 10.2 | ± 1.5 |
| Gestational age | 36.9 | ± 2.1 |
Table (2) shows the dependence of serum mercury levels in mothers and neonates on the frequency of fish consumption per month. There is highly significant difference between fish consumption per month and mercury level either in maternal serum or in their newborns cord blood serum among the three groups (P ˂0.01).
Table 2: Serum mercury levels in maternal and cord blood according to fish consumption.
| Mercury level | Fish consumption | F* | P** | ||
| none or once/ month
mean ±SD |
2 to 3 times/ month
mean ±SD |
more than 4 times/ month
mean ±SD |
|||
| Maternal Hg (μg/L) | 1.56±1.12 | 6.11±3.56 | 23.10±5.08 | 174.42 | 0.00 |
| Cord blood Hg (μg/L) | 1.58±1.20 | 6.08±3.52 | 20.55±5.55 | 123.55 | 0.00 |
*One way ANOVA, Post Hoc multiple comparisons.
**P value ˂ 0.01(highly significant), ≤ 0.05 (significant), > 0.05 (insignificant).
Table (3) illustrates a highly significant correlation (P ˂0.01) between the mercury levels in maternal blood and their fetal cord blood.
Table 3: Correlation between the mercury levels in maternal blood and cord blood.
| Mercury level | M Hg | |
| Cord blood Hg | r* | 0.948 |
| P** | 0.000 | |
*Pearson’s coefficient correlation test.
**P value ˂ 0.01(highly significant), ≤ 0.05 (significant), > 0.05 (insignificant).
Table (4) shows highly significant positive correlation (P ˂0.01) between the frequency of fish consumption and mercury level in each of maternal blood and cord blood of their newborns.
Table 4: Correlation between the frequency of fish consumption and mercury level in each of maternal blood and cord blood.
| Mercury level | fish consumption | |
| M Hg | r* | .889 |
| P** | .000 | |
| Cord blood Hg | r* | .878 |
| P** | .000 | |
*Pearson’s coefficient correlation test.
**P value ˂ 0.01(highly significant), ≤ 0.05 (significant), > 0.05 (insignificant).
Fig. (1) Box and Whisker plots present the distribution of mercury level in maternal blood according to fish consumption.
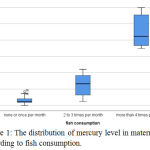 |
Figure 1: The distribution of mercury level in maternal blood according to fish consumption.
|
Fig. (2) Box and Whisker plots Illustrate the distribution of mercury level in cord blood according to fish consumption of pregnant mothers.
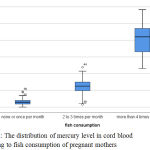 |
Figure 2: The distribution of mercury level in cord blood according to fish consumption of pregnant mothers.
|
Discussion
Mercury is ubiquitous element in the earth, it is well established known toxic trace element. Mercury toxicity is rising and spreading as a side effect of industrial development and accompanied pollution. The industrial pollution reaches the aquatic environment, and affects the fish food chains with transferred bioaccumulation [11-12].
Fish are the final link in the food chain in the aquatic environment. The existence of mercury in fish and their products is significant. Accumulation of mercury in fish muscles depends on its concentration in the environment, as it is fixed to all the organs and tissues of fish and not released [2].
The placental membrane is lipophilic, on the other side the mercury vapour and methylmercury are fat-soluble so they penetrate the placental membrane easily. Mercury has been found to lessen the carrying ability of blood to oxygen encouraging hypoxic state. In addition, mercury may affect the balance of most of the body’s essential nutrients by inhibition of nutrient transport through the placental membrane. An inhibition of nutrient transport may cause fetal death, congenital malformations, or growth retardation [4].
The data of the present study revealed highly significant difference between mercury levels either in maternal serum and their newborns cord blood among the three groups regarding the monthly frequency of fish consumption this finding is consistent with the results obtained by Song et al. [13]. In accordance to our results, Taylor et al. [14] reported that fish-eaters are significantly have high blood mercury level that markedly increased with increased frequency of consumption of fish especially white fish and of oily fish.
Basu et al. [15] assessed the mercury level in maternal blood through the three trimesters of conception and the fetal cord blood and they found that the maternal blood mercury values are not significantly different across the three trimesters, but they are significantly correlated. These investigator observed that only third trimester maternal mercury level is significantly associated to cord blood mercury which is explained as the early fetal exposure may be not well reflected in cord blood samples due to the mercury half-life in the blood is about 3-4 months. In addition they mentioned that the seafood consumption servings per month are positively and significantly correlated with maternal blood mercury in the second trimester and more in the third trimester.
Studying the coefficient relation between mercury levels in maternal blood and cord blood displayed a highly significant positive correlation. This result comes in parallel with the previous studies [3, 13, 16].
Kim et al. [16] monitored the total mercury and methylmercury levels in the maternal blood and in the umbilical cord blood at birth and they found high significant positive correlation between them which is explained by high fish and shellfish consumption.
The current study showed highly significant positive correlation between the frequency of fish consumption per month and mercury level in each of maternal blood and cord blood samples. This result is similar to that of [3, 13].
Kim et al. [3] study compared between mercury levels in two groups of pregnant women; group received fish as its usual frequency and group advised to restrict fish consumption during pregnancy (case-control study). These investigators found that the maternal blood mercury level in late pregnancy is positively correlated with mercury level of cord blood. Also, the blood mercury level in the group who ate fish more than four times per month has been found to be significantly higher than that of the group who did not consume fish. Finally the level of blood mercury has been found to be decreased in fish restricted group in comparison to the other group when these subjects are followed up for one year.
From other point of view, fish consumption during pregnancy is balanced scale between benefits and risks (hazards). Fish is known as important source of vitamins, iodine and long-chain polyunsaturated fatty acids (omega-3). It also provides necessary minerals such as manganese (Mn), copper (Cu), and selenium (Se), that may illustrate the neutralization role against toxic effects triggered by mercury [6, 17-18].
Wide disperse in the guidelines and recommendations about maternal fish consumption are present, they are relatively consistent to avoid the predatory species prevalent and consumed in each county, in addition to widening the intake spacing and decreasing the amount of serving. The widening of variation distance is depending to each country circumstances regarding aquatic pollution, type of fish available, and food costumes [1].
In summary, mercury is a cumulative harmful toxin, the industrial pollution invaded the aquatic environment, and mercury tends to accumulate in fish food chains reaching the human beings. The level of maternal blood mercury before delivery is significantly related to the mercury level of their newborns, besides the maternal and cord blood mercury levels are significantly associated with the amount of fish consumption. Contamination and fish frequency consumption are major associated risk factors for elevated mercury blood level.
Conclusion
The outcomes of the current study revealed high significant difference between mercury level either in maternal blood and their newborns cord blood among the studied groups regarding the frequency of fish consumption per month. High significant positive correlation has been found between mercury levels in maternal blood and cord blood. High significant positive correlation has been observed between the frequency of fish consumption per month and mercury level in each of maternal blood and cord blood samples. So, fish frequency consumption equilibrium is needed, biomonitoring and evaluation of contaminated regions are needed.
Acknowledgements
We are acknowledging the National Research Centre for funding our research. All appreciation to the participants mothers for their time and help.
Conflict of Interest
There is no conflict of interest.
Funding Source
This study was funded by in-house research plan of National Research centre, Dokki, Giza, Egypt. (Grant no. 11010140).
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Madical Ethical Committee of the National Research centre, Dokki, Giza, Egypt. (Grant no. 16/295).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Referances
- Taylor C. M, Emmett P. M, Emond A. M, and Golding J. A review of guidance on fish consumption in pregnancy: Is it fit for purpose? Public Health Nutr., 2018; 21(11): 2149–2159.
CrossRef - Kimáková T, Kuzmová L, Nevolná Z, Bencko V. Fish and fish products as risk factors of mercury exposure. Ann Agric Environ Med., 2018; 25(3):488-493.
CrossRef - Kim E. H, Kim I. K, Kwon J. Y, Kim S. W, and Park Y. W. The Effect of Fish Consumption on Blood Mercury Levels of Pregnant Women. Yonsei Medical Journal., 2006; 47(5):626– 633.
CrossRef - Agrawal A. Toxicity and Fate of Heavy Metals with Particular Reference to Developing Foetus. Advances in Life Sciences, 2012; 2(2): 29-38.
CrossRef - Zilversmit L, Wickliffe J, Shankar A, Taylor R. J, Harville E. W. Correlations of Biomarkers and Self-Reported Seafood Consumption among Pregnant and Non-Pregnant Women in Southeastern Louisiana after the Gulf Oil Spill: The GROWH Study. Int J Environ Res Public Health., 2017; 14(7): E784.
CrossRef - Cunha M. P, Marques R. C and Dórea J. G. Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon Nutrients. 2018; 23;10(9): E1146.
CrossRef - World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser., 1995; 854:1-452.
- World Health Organization. WHO child growth standards: length/height for age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age, methods and development. World Health Organization, Geneva, 2006. pp.xx + 312 pp. ref.40, ISBN: 924154693X.
- Apgar V, Holaday D. A, James L. S, Weisbrot I. M, Berrien C. Evaluation of the newborn infant; second report. J Am Med Assoc., 1958; 168:1985-1988.
CrossRef - Metwally F. M, Abdelraoof E. R, Rashad H, Hasheesh A, Elsedfy Z. B, Gebril O, Meguid N. A. Toxic effect of some heavy metals in Egyptian autistic children. Int. J. Pharm. Clin. Res., 2015; 7(3): 206–211.
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, Ji Y, Hong X, Caruso D, Bartell T, Gong Y, Strickland P, Navas-Acien A, Guallar E, and Wang X. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children J Expo Sci Environ Epidemiol., 2014; 24(5): 537–544.
CrossRef - Castaño A, Cutanda F, Esteban M, Pärt P, Navarro C, Gómez S, Rosado M, López A, López E, Exley K, Schindler B. K, Govarts E, Casteleyn L, Kolossa-Gehring M, Fiddicke U, Koch H, Angerer J, Den Hond E, Schoeters G, Sepai O, Horvat M, Knudsen LE, Aerts D, Joas A, Biot P, Joas R, Jiménez-Guerrero J. A, Diaz G, Pirard C, Katsonouri A, Cerna M, Gutleb A.C, Ligocka D, Reis F. M, Berglund M, Lupsa I.R, Halzlová K, Charlier C, Cullen E, Hadjipanayis A, Krsková A, Jensen J. F, Nielsen J. K, Schwedler G, Wilhelm M, Rudnai P, Középesy S, Davidson F, Fischer M. E, Janasik B, Namorado S, Gurzau A. E, Jajcaj M, Mazej D, Tratnik J. S, Larsson K, Lehmann A, Crettaz P, Lavranos G, Posada M. Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environ Res., 2015; 141:58-68.
CrossRef - Song Y, Lee C, Kim K, Lee J, Suh C, Kim S, Kim J, Son B, Kim D, Lee S. Factors associated with total mercury concentrations in maternal blood, cord blood, and breast milk among pregnant women in Busan, Korea. Asia Pac J Clin Nutr., 2016; 25(2):340-349.
- Taylor C. M, Golding J, Emond A. M. Blood mercury levels and fish consumption in pregnancy: Risks and benefits for birth outcomes in a prospective observational birth cohort. International Journal of Hygiene and Environmental Health. 2016; 219(6):513-520.
CrossRef - Basu N, Tutino R, Zhang Z, Cantonwine D. E, Goodrich J. M, Somers E. C, Rodriguez L, Schnaas L, Solano M, Mercado A, Peterson K, Sánchez B. N, Hernández-Avila M, Hu H, Maria Téllez-Rojo M. Mercury levels in pregnant women, children, and seafood from Mexico City. Environ Res., 2014; 135:63-69.
CrossRef - Kim Y, Chung J, An H, Park S, Kim B, Bae J, Han M, Cho Y, and Hong Y. Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea. Int. J. Environ. Res. Public Health. 2015; 12: 13482-13493.
CrossRef - Oken E, Radesky J.S, Wright R.O, BellingerD. C, Amarasiriwardena C. J, Kleinman K. P, Hu H, and Gillman M. W. Maternal fish intake during pregnancy, blood mercury, and child cognition at age 3 years in a US cohort. Am J Epidemiol., 2008; 15; 167(10): 1171–1181.
CrossRef - Huang S. H, Weng K. P, Ger L. P, Liou H. H, Lin C. C, Wang C. C, Lee C. T, Wu M. T. Influence of seafood and vitamin supplementation on maternal and umbilical cord blood mercury concentration. J Chin Med Assoc., 2017; 80(5):307-312.
CrossRef







