Manuscript accepted on :12-Mar-2019
Published online on: 22-03-2019
Plagiarism Check: Yes
Reviewed by: Nur Atik
Second Review by: Irma Melyani Puspitasari
Final Approval by: Dr. H Fai Poon
Andri Rezano*1,2 , Thifal Indra Zhalfani Siregar3, Adi Santosa Maliki1, Melia Juwita Adha4
, Thifal Indra Zhalfani Siregar3, Adi Santosa Maliki1, Melia Juwita Adha4 and Listya Hanum2
and Listya Hanum2
1Department of Biomedical Sciences, Division of Cell Biology, Faculty of Medicine Universitas Padjadjaran, Sumedang, Indonesia.
2Graduate School of Biomedical Sciences Master Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
3Faculty of Medicine, Universitas Padjadjaran, Sumedang, Indonesia.
4Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin Hospital, Bandung, Indonesia.
Corresponding Author E-mail: andri.rezano@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1650
Abstract
Caesalpinia sappan has been studied for its biological activities in the wound healing process. Therefore, this study is conducted to investigate the wound healing activity of sappanwood extract in an excisional wound of Balb/c mice. Animal experiments were designated with, positive control group, negative control group, and three groups of treatment (G1, G2 and G3). The groups consist of povidone iodine ointment, blank gel, 5%, 30%, 90% concentration of sappanwood extract gel, respectively. All groups were applied topically on the surface of the biopsy wound once daily for 10 days. The wounds of the 2nd, 8th and 10th day of treatment were observed and measured. There was a significant difference from each group on day 2, 6 and 10 after wound induction (p=0,001, p=0,001 and p=0,006). The tendency of wound closure happened in positive group control, negative group control and G1. Negative control group showed the highest percentage of wound closure amongst all groups. Meanwhile, in G2 and G3 showed less percentage of closure and healing compared to the others. Despite there is a tendency of ethanolic C. sappan extract gels to the wound healing, administration of C. sappan extract gels topically do not heal the skin excisional wound.
Keywords
Caesalpinia Sappan; Mice Model; Sappanwood Extract Gel; Wound Healing
Download this article as:| Copy the following to cite this article: Rezano A, Siregar T. I. Z, Maliki A. S, Adha M. J, Hanum L. Sappanwood (Caesalpinia sappan) Extract Gel Do Not Heal Skin Excisional Wound on Balb/c Mice. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Rezano A, Siregar T. I. Z, Maliki A. S, Adha M. J, Hanum L. Sappanwood (Caesalpinia sappan) Extract Gel Do Not Heal Skin Excisional Wound on Balb/c Mice. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2CwYuPX |
Introduction
A wound caused by injury is the most frequent happens to any human. Wound healing consists of four highly integrated and overlapping phases that elicit right after the injury happens. There are hemostasis, inflammation and tissue remodeling or resolution.2 Healing of wound that does not progress in a timely and orderly manner that could happen because of a delayed, incomplete or uncoordinated healing process can convert into the chronic wound which frequently enters a pathologic inflammation state.2,3 Such wounds require the patients to live in pain, emotional problems and social isolation. Moreover, the treatment demands multiple times of visits or weekly dressings in the clinic.4 These can decrease one’s productivity and can be an economic burden for the wound’s care.1 There were an estimated 2.2 million wounds that cost £4.5–5.1 billion for managing them in the UK between the year of 2012/2013.5
Studies on medicinal plants confirmed that herbal drugs exhibit fewer side effects in comparison with chemical agents, and are more cost-effective. Just 1% – 3% of chemicals listed in Western pharmacopeia are indicated for treating wounds and skin disease, while more than 30% of herbal medicaments are considered beneficial.6
Caesalpinia sappan or known as secang in Indonesia is a flowering tree from Leguminosae family which wide-distributed throughout Southeast Asia. In Indonesia, its heartwood is traditionally used for skin care.7 It is stated that in India, the wood of C. sappan is used in toothpaste as a component due to its strong healing action to stop bleeding in gums.8
Brazilin is the main flavonoid found in sappanwood which its extracts have been known for various biological activities. A study of brazilin rich extract (BRE) in wound healing activity using human fibroblast in-vitro scratch assay shows the percentage migration was almost doubled that in the control group. In the same study also shows the anti-oxidant and anti-bacterial activity of BRE and brazilin which contribute to wound healing activity. 9,10
There has been no report on wound healing activity of C. sappan extract on an in-vivo study. Therefore, this study was conducted to investigate the wound healing activity of sappan wood extract in excisional wound of Balb/c mice.
Material and Methods
Research Subject and Design
The laboratory experimental was analytic research by quantitative method. The subject of this research was an animal experiment using 32 male mice strain Balb/C obtained from Bio Farma with age 7-8 weeks and weight 22 – 40 grams which adapted to the laboratory for 14 days. Adaptation and experiment processes were conducted in Biochemistry Laboratories, Faculty of Medicine, Universitas Padjadjaran.
This study has obtained ethical approval from Health Research Ethics Committee, Faculty of Medicine, Universitas Padjadjaran (No. 597/UN6.KEP/EC/2018).
The subjects were divided into 5 groups of mice, including positive control group, negative control group, G1, G2, and G3 which given povidone iodine ointment, blank gel, 5%, 30%, 90% concentration of sappanwood extract gel, respectively. All groups were given topically on the surface of the biopsy wound once daily for 10 days.
Gel Material
Sappanwood extract gel was prepared by mixing Caesalpinia sappan 70% ethanol extract into a carbopol-based gel containing 5% Carbomer 934 in 100 ml and 50 ml in 90% concentration gel. While the blank gel only consists of the carbopol gel. All gels were prepared in Pharmaceutical Laboratory, Faculty of Pharmacy, Universitas Padjadjaran.
Induction of Wound and Gel Administration
Initially, mice were anesthetized with ketamine injection then its back hair was shaved to clear the area of wound induction. Aseptic and antiseptic step was performed using alcohol 70%. Wound induction was made using 6 mm sterile biopsy punch to achieve 6 mm in diameter circular-shaped and 2 mm depth of wound extending up to adipose tissue. Wounds were left open and treated with gel on its surface soon after wound induction was made.
Experiment occurred for 10 days. On the day-0 of the experiment, 2 mice from each group were inducted. Wound induction was done again in day-4 of the experiment for 2 mice from each group, except G2 and G3 which takes 3 mice. The rest of the mice was inducted on the day-8 of the experiment. These were done to achieve the day of 10th, 6th and 2nd of the treatment so that all of the mice’s skin tissue could be collected in the final day of the experiment.
Wound Measurement
Wound area was measured at 2nd, 6th and 10th days of treatment. Analyzing the wound area was done using Image J (open source) application. The area obtained then calculated to be the percentage of the wound closure using the formula below:
![]()
Initial wound closure (in day 0) was defined as 0% wound closure.11
Histological Study
The skin tissue consisting the wound was taken, paraffin formalin-fixed and prepared as a histological sample with hematoxylin-eosin (HE) staining and observed under light microscope. Comparison of wound healing processes between groups was made.
Statistical Analysis
Shapiro-Wilk was used in order to know normality of the data distribution and homogeneity test using Levene test. ANOVA then used when data was normal distributed and homogen, followed by multiple comparisons post-hoc Tukey. Whereas Kruskal-Wallis test was used for abnormal distribution data. A p-value of <0.05 was regarded as significant.
Result and Discussion
The Development of the Percentage of Wound Closure Between Control and Treatment Groups.
The development of the percentage of wound closure among groups on day 2, 6, and 10 as shown in Graph 1.
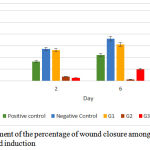 |
Graph 1: The development of the percentage of wound closure among groups on day 2, 6 and 10 after wound induction.
|
There was a significant difference between each group on day 2, 6 and 10 after wound induction (p=0,001, p=0,001, and p=0,006). As indicated in Figure 1, the tendency of wound closure happens in positive control group, negative control group and G1. Negative control group shows the highest percentage of wound closure amongst all groups. Meanwhile in G2 on day 6 shows a reduction of percentage indicating the wound is enlarged as well as in G3 on day 10.
Figure 1 presents the representative images of wound closure progress from each group.
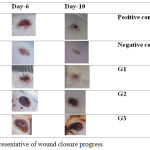 |
Figure 1: The representative of wound closure progress.
|
The histological images of positive control group, negative control group and G1 are shown in Figure 2.
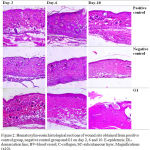 |
Figure 2: Hematoxylin-eosin histological sections of wound site obtained from positive control group, negative control group and G1 on day 2, 6 and 10. E-epidermis; DL-demarcation line; BV-blood vessel; C-collagen; SC-subcutaneous layer. Magnifications (x10).
|
Histological Observation of Wound Tissues in Control and Treated Groups 2 Days Post Wound Induction
All groups showed inflammation process marked with blood vessels appearance which G1 has the most abundantly. They are also showed thickened epidermis. Demarcation line which separates the necrosis and vital tissue were seen clearly in negative control group and G1.
6 days post wound induction
Blood vessels in positive control group were seen to still have more than the other groups. There was a decrease in number in the negative control group while very few in G1 which indicate inflammation phase was almost finished. It also can be seen newly created granulation tissue at the bottom of wounds and synthesized non-organized collagen in the dermis layer.
10 Days Post Wound Induction
Compared to the other two, negative control group has the finest arrangement of wound healing. The collagen dominated the dermis with more organize arrangement rather than in G1. The inflammatory phase in negative control group was finished by the absence of blood vessel. While there was some blood vessels and delayed collagen synthesis observed in positive control group.
Based on observation of the excisional wound closure, there were significant differences among groups on day 2, 6 and 10. This phenomenon can be explained by understanding the mechanism of wound healing and sappanwood intervention to the healing process.
Wound healing consists of four highly integrated and overlapping phases consists of hemostasis, inflammation and tissue remodeling or resolution. The first phase of hemostasis begins immediately after wounding, with vascular constriction and fibrin clot formation. The clot and surrounding wound tissue release pro-inflammatory cytokines and growth factors to migrate inflammatory cells into the wound (chemotaxis) and promote the inflammatory phase once bleeding is controlled.2
The proliferative phase generally follows and overlaps with the inflammatory phase, and is characterized by epithelial proliferation and migration over the provisional matrix within the wound (re-epithelialization). Fibroblasts and endothelial cells are the most prominent cell types in reparative dermis at the site of injury to present and support capillary growth, collagen formation, and the formation of granulation tissue. Fibroblasts produce collagen, glycosaminoglycans, and proteoglycans, which are major components of the extracellular matrix (ECM) within the wound bed.2
The remodeling phase is when the ECM undergoes remodeling to a normal tissue architecture also when the wound undergoes physical contraction throughout the entire wound healing process, which is believed to be mediated by contractile fibroblasts (myofibroblasts) that appear in the wound.2
Several studies have been conducted to explore wound healing activity of C. sappan extract and its main phenolic compound, brazilin.9,10,12
Brazilin as an active substance of C. sappan has various mechanisms to wound healing process. A study conducted by Supinya showed that ethanolic C. sappan extract enhanced fibroblast proliferation more than brazilin. In the same study also stated the cellular proliferation and migration of L929 fibroblast cells as each edge of the gaps moved toward each other and to close the scratch area.12
Antiinflamation activity of brazilin against inflammatory mediators PGE2 and TNF-α were reported. In addition, the same study showed the down-regulation of mRNA expressions of the iNOS and COX-2 genes as the antiinflammatory mechanism of brazilin.12
Previous study evaluated brazilin and brazilin rich extract (BRE) antibacterial activity was most active against S. aureus as compared to P. aeruginosa. This activity would avoid infection in the wound area in order to help wound healing process. 10
However, the result of this study does not indicate that sappanwood extract helps the wound healing processes. This can be caused by many factors including the composition and/or the balance of the extract, water or carbomer of the gel itself. Besides, moisture effect on wound healing plays a big role in this study. The blanko gel used in negative control group was the moistest gel compared to the others since the more concentration of the extract contained in gels the less water concentration they had.
The moist environment provides wound to heal faster, enhance angiogenesis and collagen synthesis, and prevent dehydration cell that can cause crust forming over the wound.13,14 These could be attributed to easier migration of epidermal cells, faster epithelization also prolonged presence of proteinases and growth factors provided on moist dressing wound. A study showed that inflammatory and proliferative phases of dermal repair were shorter and concluded that the rate of revascularized was greater in moist wound environment than those maintained under dry condition. Also angiogenesis occured in a more orderly fashion in moist wounds.14
Conclusion
In conclusion, despite there is a tendency of ethanolic C. sappan extract gels to the wound healing, administration of C. sappan extract carbopol-based gels ranging 5-90% in concentration do not heal the skin excisional wound topically.
Acknowledgements
This study was supported by Internal University Grant Scheme of Universitas Padjadjaran, Ministry of Research, Technology and Higher Education of the Republic of Indonesia. The authors would like to acknowledge Mr. Asep Saepuloh and all staff of Animal Laboratory, Faculty of Medicine Universitas Padjadjaran and Mr. Deni Rusyandi and all staff of Pharmaceutical Laboratory, Faculty of Pharmacy Universitas Padjadjaran for all helps and guidance in order to accomplish this project.
Conflict of Interest
There is no conflict of interest.
References
- Sen C. K., Gordillo G. M., Roy S., et al. Human Skin Wounds: A Major Snoballing Threat to Public Health and Economy. Wound Repair Regen 2009. 2010;17(6):763-771. doi:10.1111/j.1524-475X. 2009.00543.x. Human.
- Guo S., DiPietro L. A. Critical review in oral biology & medicine: Factors affecting wound healing. J Dent Res. 2010;89(3):219-229. doi: 10.1177/0022034509359125.
CrossRef - Velnar T., Bailey T., Smrkolj V. The Wound Healing Process : an Overview of the Cellular and Molecular Mechanisms. 2009;37(5):1528-1542. doi: 10.1177/147323000903700531.
CrossRef - Vowden P. Hard- to- heal Wounds. Wound Int. 2011;2(4):1-6. http://www. wound si nternational.com/media/issues/514/files/content_10140.pdf.
- Guest J. F., Ayoub N., McIlwraith .T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12). doi: 10.1136/bmjopen-2015-009283.
CrossRef - Farzaei M. H., Zabra a., Mohammad R. S., Abdollahi M., Rahimi R. A comprehensive review of plants and their active constituents with wound healing acttivity in traditional Iranian Medicine. Wounds. 2014;26(7):197-206.
- Batubara I., Mitsunaga T., Ohashi H. Brazilin from Caesalpinia sappan wood as an antiacne agent. J Wood Sci. 2010;56(1):77-81. doi: 10.1007/s10086-009-1046-0.
CrossRef - Badami S., Moorkoth S., Suresh B. Caesalpinia Sappan A Medical and Dye Yielding Plant. Nat Prod Radiance. 2004;3(2):75-82.
- Nirmal N. P., Rajput M. S., Prasad R. G. S. V., Ahmad M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: A review. Asian Pac J Trop Med. 2015;8(6):421-430. doi:10.1016/j.apjtm. 2015.05.014.
CrossRef - Nirmal N. P., Prasad R. G. S. V., Keokitichai S. Wound healing activity of standardized brazilin rich extract from Caesalpinia sappan heartwood. J Chem Pharm Res. 2014;6(10):195-201.
- Berbudi A., Hasibah U. H., Faried A., Putri A. C HME. Administration of Curcuma longa Extract Topically does not Accelerate Skin Excision Wound Closure in Alloxan-induced Diabetic Mice Model. Biomed Pharmacol J. 2018;11(1).
- Tewtrakul S., Tungcharoen P., Sudsai T., Karalai C., Ponglimanont C., Yodsaoue O. Antiinflammatory and wound healing effects of Caesalpinia sappan L. Phyther Res. 2015;29(6):850-856. doi:10.1002/ptr.5321.
CrossRef - Hess C. T. Practice Points Checklist for Factors Affecting Wound Healing. 2008;24(4):17112.
- Junker J. P. E., Kamel R. A., Caterson E. J., Eriksson E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet and Dry Environments. 2013;2(7):348-356. doi:10.1089/wound.2012.0412.
CrossRef







