Manuscript accepted on :11-Feb-2019
Published online on: 18-03-2019
Plagiarism Check: Yes
Reviewed by: Md. Sarwar Hossain
Second Review by: Muhammad Shahzad Aslam
Final Approval by: Dr. Ayush Dogra
N. Venkata Raju1, Karuganti Sukumar2, G. Babul Reddy3, P. K. Pankaj4, Muralitharan G2 , Shanti Annapareddy1, D. Teja Sai1 and Anjani Devi Chintagunta*1
, Shanti Annapareddy1, D. Teja Sai1 and Anjani Devi Chintagunta*1
1Vignan's Foundation for Science, Technology and Research, Vadlamudi, Guntur, 522213, India.
2Department of Microbiology, Bharathidasan University, Tiruchirapalli, Tamilnadu, 620024, India.
3R and D Center, SOM Phytopharma (India) Limited, Hyderabad,502325, India.
4ICAR-Central Research Institute for Dryland Agriculture, Hyderabad, 500059, India.
Corresponding Author E-mail: sumapriya.ch@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1648
Abstract
Mangifera indica L. belongs to the family Anacardiaceae and is considered as “King of all Fruits”. Mango kernels are discarded as waste after the industrial processing and it has several proven medicinal benefits. Attempts were made to study its antitumour and antimicrobial activities. In the current research work, 15 local cultivars of Mangifera indica L. were collected with a motto to screen the best cultivar having high total phenolic content, flavonoid content, antitumour property and antimicrobial activity. Banganapalli cultivar of mango showed high total phenolic content and total flavonoid content i.e. 63.5±1.1 mg GAE/g and 16.7±0.5 mg quercetin/g followed by Royal special cultivar (TPC-58.7±0.6 mg GAE/g TFC-16.2±0.6 mg quercetin/g). Mangifera indica L. cultivar Banganapalli which showed highest total phenolic content and total flavonoid content was screened for its antitumour and antimicrobial properties. Antitumour property was tested by using potato disc assay which recorded 40.12% tumour inhibition percentage. Antimicrobial activity was assessed by agar diffusion method by taking 3 test microorganisms viz. Bacillus subtilis subsp. subtilis DSM 10, Staphylococcus aureus MTCC 737 and Escherichia coli MTCC 46. The measured area of inhibition is around Staphylococcus aureus MTCC 737 in 8.5±0.3 mm followed by E.coli MTCC 46 (8.2±0.3 mm) and Bacillus subtilis sub subtilis (6.6±0.5 mm). The present study showed that the mango kernels which were generally discarded as waste has antitumour and antibacterial properties and further studies need to be carried out.
Keywords
Antimicrobial Activity; Antitumour Activity; Banganapalli; Kernels; Mangifera Indica L.
Download this article as:| Copy the following to cite this article: Raju N. V, Sukumar K, Reddy G. B, Pankaj P. K, Muralitharan G, Annapareddy S, Sai D. T, Chintagunta A. D. In-Vitro Studies on Antitumour and Antimicrobial Activities of Methanolic Kernel Extract of Mangifera Indica L. Cultivar Banganapalli. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Raju N. V, Sukumar K, Reddy G. B, Pankaj P. K, Muralitharan G, Annapareddy S, Sai D. T, Chintagunta A. D. In-Vitro Studies on Antitumour and Antimicrobial Activities of Methanolic Kernel Extract of Mangifera Indica L. Cultivar Banganapalli. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2Jlmnjv |
Introduction
From the time immemorial, herbal plants and their by-products have been used widely for treatment of several diseases.1 In the recent years, herbal medicine is gaining much importance all over the world because of its natural origin and less side effects.2 As per WHO reports, 80% of the world’s population depends mostly on traditional remedies which mostly include active ingredients of plants.3 Similarly, high intake of vegetables and fruits lower the degenerative diseases like cardiovascular diseases and cancer.4
Cancer is considered as one of the deadly diseases in the world which is characterized by continues proliferation of abnormal cells which is indicated by damage of the surrounding tissues.5 Synthetic medicines used for curing the diseases have toxic and dreadful effects due to which much attention has been drawn towards the herbal medicines.6 Research reports show nearly 1400 genera of herbal plants with a potential to treat cancer.7
Mangifera indica L. belongs to the family Anacardiaceae that includes above 70 genera. Annually, 1 million tones of mango seeds are being wasted. Beneficial utilization of these seeds to produce commercially valuable products may eliminate some of the hazardous compounds.8 As per the historical records, it is originated from India and has been cultivated as fruit tree from the last 4000 years and it is also considered as “King of all fruits”.9,10 Mango is a abundant source of anti – oxidants, phenolic compounds and carotenoids.11 Poly phenols and carotenoides present in Mangifera indica L. may protect from cancer. Characterization of polyphenolic compounds shows flavonoids like quercetin, kaempferol, phenolic acids, galloyl glycosides, abundant amounts gallic acid and mangiferin.12 Quercetin which is a common flavonoid acts against different cancers by several cell signaling mechanisms and its capacity to inhibit enzymes which are majorly responsible for activation of carcinogens. Quercetin also binds to cellular receptors and proteins and thus inhibiting the cancer.13,14 Antioxidants present in mango reduce clinical toxicity induced by the cancer drugs in patients.15
Mango seed kernel weigh 15-20% of total fruit weight which is abundant source of polyphenols, antioxidants, flavonoids etc,.16 Large amount of mango seed kernels are discarded as waste after industrial processing.17 But historic reports show usage of mango kernels as a traditional medicine in almost all parts of the world. In India dry powder of kernel is applied to treat dandruff of hair and also used as to treat diarrhea. Similarly, traditional practice in Fiji includes consumption of fresh kernel for treatment of asthma and dysentery.18 Extract of kernel has been studied for its anticancer activity and showed positive results.19 In Andhra Pradesh, more than 34 local varieties of mango were identified and characterized.20
Potato disc bioassay is mostly used for testing the antitumour property which was developed based on tumour inducing property of Agrobacterium tumifaciens on potato disc. This test was mostly employed as the tumourogenic mechanism of animals and A.tumifaciens is similar.5,21
In the current study different local cultivars of mangoes were collected and its Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) were estimated in the methanolic kernel extract. Further, cultivar showing high TPC and TFC in the methanolic kernel extract was tested for antitumour and antimicrobial activities.
Materials and Mehods
Chemicals and Reagents
All the chemicals and reagents required for the experiment were procured from Merck, Bengaluru, India. Deionized water and methanol were procured from Fisher scientific, Mumbai, India.
Plant Mmaterial Collection
Plant materials of different local mango cultivars of Andhra Pradesh were collected from the Chiruvolu Lanka village, Krishna District, Andhra Pradesh (16°02’54.3”N and 80057’08.3”E) in the month of May, 2018.
Bacterial Cultures
Agrobacterium tumifaciens MTCC 609, Staphylococcus aureus MTCC 737 and Escherichia coli MTCC 46 were procured from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India. Similarly, Bacillus subtilis subsp. subtilis DSM 10 was procured from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Germany. Bacterial cultures were grown on Luria Bertani agar medium with composition g/L: Peptone – 10g, Yeast extract – 10g, NaCl – 5g, Agar – 20g.Pure cultures were grown in Luria Bertani broth and used for further studies.21
Methanolic Extraction of Mango Kernels
Collected mango kernels were washed with water to remove the pulp attached to it and dried in hot air oven at 45°C for 2 days. Dried kernels were powdered and mixed in methanol in the ratio 1:5 and kept on orbital shaker at 150 rpm for 2 days. Mixture was filtered by using Whatman No.1 filter paper and filtrate was dried in water bath at 45°C till aqueous slurry is obtained. The extract was stored at 4°C for further studies to be carried out.22
Estimation of Total Phenolic Content
Phenolic content was estimated by using modified procedure of Folin-Ciocalteu.23 0.5 ml of methanolic kernel extract was mixed in 0.5 ml of Folin–Ciocalteu reagent and 7.5 ml deionized water. Mixture was kept at room temperature for 10 min which was followed by addition of 1.5 ml of 20% sodium carbonate (w/v). The mixture was heated in water bath at 40°C for 20 min followed by rapid cooling. Absorbance was recorded at 755 nm using UV – Vis Spectrophotometer (Elico SL 244). Gallic acid was taken as the standard for estimation of total phenolic content and values were noted as milligrams of Gallic acid equivalents per 1 g.
Estimation of Flavonoid Content
Flavonoid content was estimated by taking 0.25 ml of mango kernel extract and was mixed with 1.25 ml of deionized water. 75 µL of 5% NaNO2 solution and 150 µL of 10% AlCl3.6H2O solution were added to the mixture and kept aside for 5 min followed by addition of 0.5 ml of 1 M NaOH. The reaction mixture was made to 2.5 ml by using deionized water. Absorbance was noted at 510 nm by using UV-Vis Spectrophotometer (Elico SL 244).Quercetin was taken as standard for estimation of flavonoid content.24
Testing of Antitumour activity by Potato Disc Assay
Antitumour properties of methanolic Kernel extract of mango was assessed by using potato disc assay. Potatoes (Solanum tuberosum L.) were purchase from local market, cleaned with tap water and sterilized distilled water. Cleaned potatoes were surface sterilized with 0.1% HgCl2). From the center of surface sterilized potatoes small pieces of 5mm x 8mm size were cut by using sterilized cork borer. Pieces were washed with sterilized double distilled water and transferred to water agar plates (15 g/L). Sterilized potato pieces were inoculated with 50 µL of Agrobacterium tumefaciens (1.0×109 CFU/ml). Camptothecin was used as positive control to test the antitumour activity. Petri plates were sealed with parafilm and incubated at 28°C in dark for 21 days. After incubation the pieces were stained with Lugol’s Iodine i.e. 10% KI and 5% I2.21,25 Lugol’s reagent reacts with the starch content of potato and turns to dark blue to dark brown colour, whereas tumours will not stain and appear creamy to orange5. Percentage of inhibition was calculated based on the formula:

Experiment was carried out in triplicates and the average values were taken. For testing antitumour property of plant extracts, 20% of tumour inhibition was considered as significant.1
Testing of Antibacterial Activity
Antibacterial activity of methanolic extract of mango kernels was tested against Staphylococcus aureus MTCC 737, Bacillus subtilis subsp. subtilis DSM 10 and Escherichia coli MTCC 46 by agar diffusion method. Muller Hinton agar medium was poured in 90 mm petri dishes and allowed to solidify. 100 μL of specific bacterial culture was spread uniformly on the agar surface. Wells were made by using sterile cylinder of 6mm diameter. 200 μL of fillter sterilized methanolic extract of mango kernels was added into the agar wells. Streptomycin was used as positive control and saline as negative control.Agar plates were incubated at 37°C for 24 h and zone of inhibition was measured.26
Results and Discussion
In the current research work attempts have been made to study the antitumour and antimicrobial properties of mango kernels which are generally disposed after the industrial processing. Kernel extract of Mangifera indica L. showed cytotoxicity towards the carcinogenic breast cancer cell lines.19,28,29
15 local varieties of mango viz. Cherukurasam, Chinnarasam, Hyder, Imampasand, Nalla rasalu, Nalla Andrews, Suvarnarekha, Paparao Goa, Peddarasam, Royal special, Tella Gulabi, Banganapalli, Jalal, Panchadara kalasam, Jahangir were collected from the mango gardens in Chiruvolu Lanka Village, Krishna District, India. Kernel methanolic extraction was done for all the collected local cultivars with an aim to assess the total phenolic content, total flavonoid content, antitumour and antimicrobial properties.
Total phenolic content was assessed in all the Mangifera indica L. varieties. Reports indicate total phenolic content in Mangifera pajang kernels as 103.30 ± 0.63 mg GAE/g which was also equal to Mangifera indica.4 Similarly, the total phenolic content in Langra mango and Chonsa mango were recorded to be 63.89±0.72 and 69.24±0.54 respectively.27 All the local varieties of Mangifera indica L. were tested for total phenolic content among which Banganapalli showed highest total phenolic content with 63.5±1.1 mg GAE/g followed by Royal special (58.7±0.6 mg GAE/g) and Tella Gulabi (56.5±0.7 mg GAE/g) (Fig.1).
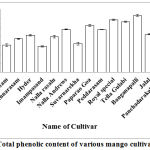 |
Figure 1: Total phenolic content of various mango cultivars.
|
Quercetin was taken as reference standard for estimation of total flavonoid content as it has the anticancer property to inhibit the breast cancer cells13.When total flavonoid content was estimated, Banganapalli recorded highest value 16.7±0.5 mg quercetin/g followed by Royal special (16.2±0.6 mg quercetin/g) and Paparao Goa (15.1±0.5 mg quercetin/g) respectively (Fig 2). Banganapalli variety of mango that showed highest total phenolic content and total flavonoid content was tested for its antitumour and antibacterial properties.
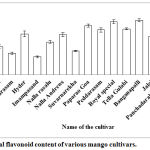 |
Figure 2: Total flavonoid content of various mango cultivars.
|
Antitumour property of methanolic kernel extract was assessed by potato disc assay in which camptothecin was taken as positive control. Results recorded were satisfactory with 40.12% of tumour inhibition whereas in positive 100% tumour inhibition was noticed. Above 20% of tumour inhibition was considered as significant.1
Antimicrobial activity of Mangifera indica L. methanolic kernel extract was done and the results obtained were encouraging. Among all the tested microorganisms, highest zone of inhibition was recorded in Staphylococcus aureus MTCC 737 with 8.5±0.3 mm followed by E.coli MTCC 46 (8.2±0.3 mm) and Bacillus subtilis subsp. subtilis DSM 10 (6.6±0.5 mm) (Fig.3).
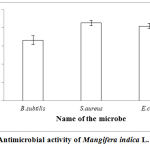 |
Figure 3: Antimicrobial activity of Mangifera indica L.
|
Conclusion
Mangifera indica L. cultivar Banganapalli kernels which were generally considered as waste showed encouraging results of antitumour and antimicrobial activities. This study is an attempt to test the antitumour and antimicrobial activities of kernel extracts and further in depth research studies needs to be carried out for testing the anticarcinogenic activity.
Acknowledgements
We wish to express our gratitude to Vignan’s Foundation for Science, Technology & Research, Vadlamudi, Guntur, 522213, India for supporting the research work.
Conflict of Interest
There is no conflict of interest.
References
- Yildirim A. B., Karakas F. P and Turker A. U. In vitro antibacterial and antitumour activities of some medicinal plant extracts, growing in Turkey. Asian Pacific Journal of Tropical Medicine. 2012;616-624.
- Parve G. M. M. Pharmacological Activities of Mango (Mangifera indica): A Review. Journal of Pharmacognosy and Phytochemistry. 2016;5(3):01-07.
- Ahmad I., Mehmood Z and Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. Journal of Ethnopharmacology. 1998;62:183–193.
CrossRef - Bakar M. F. A., Mohamed M and Rahmat A., Fry J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus).Food Chemistry. 2009;113:479–483.
CrossRef - Islam M. S., Akhtar M. M., Rahman M. M., Rahman M. A., Sarker K. K and Alam M. F. Antitumour and Phytotoxic Activities of Leaf Methanol Extract of Oldenlandia diffusa (Willd.) Roxb. Global Journal of Pharmacology. 2009;3(2):99-106.
- Harun – ur – Rashid., Gafur M. A., Md. Sadik G and Md. Rahman A. A. Biological activities on a new Acrylamide derivative from Ipomea turpethum. Pakistan Journal of Biological sciences. 2002;5(9):968-969.
CrossRef - Parvez G. M. M and Mosaddik A. Evaluation of anticancer property of mango peel and flesh after formalin treatment. The Journal of Phytopharmacology. 2016;5(3):112-116.
- Abdalla E. M. A.,Darwish M. S., Ayad H. E. E., Reham M and El-Hamahmy. Egyptian mango by-product 1. Compositional quality of Mango seed Kernels. Food Chemistry. 2007;103:1134–1140.
CrossRef - Ribeiro M. S M. R and Schieber A. Bioactive Compounds in Mango (Mangifera indica), Bioactive Foods in Promoting Health: Fruits and Vegetables. 2010;34:507-523.
- Jagarlamudi S., Rosaiah G., Kurapati K. R & Pinnamaneni R. Molecular identification of Mango, Mangifera indica var. totupura. Bioinformation. 2011;5(10):405-409.
CrossRef - Ribeiro S. Y. R., Barbosa L. C. A., Queiroz J. H., Knodler M., Schieber A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L .) varieties. Food Chemistry. 2008;110:620–626.
CrossRef - Noratto D. G., Bertoldi C. M.,Krenek K.,Talcott T. S., Stringheta C. P and Mertens-Talcott U. S., Anticarcinogenic Effects of Polyphenolics from Mango (Mangifera indica) Varieties. Agric. Food Chem. 2010;58:4104–4112.
CrossRef - Wang R., Yang L., Li S.,Ye D.,Yang L., Liu Q.,Zhao Z.,Cai Q.,Tan J., Li X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med Sci Monit. 2018;24:412-420.
CrossRef - Khan F., Niaz K., Maqbool F., Hassan I. F.,Abdollahi M., Venkata N. C. K., Nabavi M. S and Bishayee A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update, Nutrients. 2016;8:529. doi:10.3390/nu8090529.
CrossRef - Udem C. G., Dahiru D., Etteh C. C. In vitro Antioxidant Activities of Aqueous and Ethanol Extracts of Mangifera indica Leaf, Stem-Bark and Root-Bark. Commn. 2018;8(3):119-124.
CrossRef - Jahurul H. A., Zaidul I. S. M.,Beh L., Sharifudin M. S., Siddiquee S., Hasmadi M., Sahena F.,Mansoor A. H., Lee J. S., Jinap S. Valuable components of bambangan fruit (Mangifera pajang) and its co-products: A review. Frin. 2018. doi: 10.1016/j.foodres. 2018.08.017.
- Correia B. L., Fiuza A. R. Jr., de Andrade C. R., Andrade M. C. H. CO2 capture on activated carbons derived from mango fruit (Mangifera indica) seed shells. J Therm Anal Calorim. 2017. doi: 10.1007/s10973-017-6542-7.
CrossRef - Barreto C. J.,Maria T. S. Trevisan William E. Hull,Gerhard Erben, Edy S. De Brito, Beate Pfundstein, Gerd Wu Rtele, Bertold Spiegelhalder and Robert W. Owen,Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves and Peel of Mango (Mangifera indica ). J. Agric. Food Chem. 2008;56:5599–5610.
CrossRef - Al-Shwyeh Abdullah H., Mohammed S. A., Abdullah R., Mirghani S. E. M and Al-Qubaisi M. Cytotoxic effects of Mangifera indica kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complementary and Alternative Medicine. 2014;14:199.
CrossRef - Himabindu., Srihari D., Rajasekhar M., Sudhavani V., Subbarammamma P and Krishna U. K. Characterization and Quality Assessment of Potential Indigenous Mango (Mangifera indica L.) Cultivars of Coastal Districts in Andhra Pradesh, India by Bio-Chemical Markers. Int.J.Curr.Microbiol.App.Sci. 2018;7(5):2141-2151.
- Islam M. S., Rahman M. M., Rahman M. A., Qayum M. A and Alam M. F. In vitro evaluation of Croton bonplandianum as potential antitumour properties using Agrobacterium tumefaciens. Journal of Agricultural Technology. 2010;6(1):79-86.
- Islam S., Akhtar M. M., Md. Parvez S., Md. Alam J and Alam M. F. Antitumour and Antibacterial activity of a crude Methanol leaf extract of Vitex negundo L. Arch. Biol. Sci.Belgrade. 2013;65(1):229-238.
CrossRef - Chaovanalikit and Wrolstad R. E. Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. Journal of Food Science. 2004;69:1.
CrossRef - Dewanto V., Wu X., Adom K. K and Liu H. R. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. Agric. Food Chem. 2002;50:3010-3014.
CrossRef - Galsky G. A and Wilsey P. J. Crown Gall Tumour Disc Bioassay. Plant Physiol. 1980;65:184-185.
CrossRef - Masibo M and He Q. Mango Bioactive Compounds and Related Nutraceutical Properties—A Review. Food Reviews International. 2009:25:346–370.
CrossRef - Sultana B., Hussain Z., Asif M and Munir A. Investigation on the Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera indica ). Journal of Food Science. 2012;77:8.
CrossRef - Sravani D., Aarathi K., Kumar S. N. S., Krupanidhi S.,Ramu V. D.,Venkateswarlu T. C. In Vitro Anti- Inflammatory Activity of Mangifera indica and Manilkara zapota Leaf Extract. Research J. Pharm. and Tech. 2015;8(11):1477-1480.
CrossRef - Naidu K. N., Ramu V.,Kumar S. N. S. Anti-inflammatory and anti-helminthic activity of ethanolic extract of Azadirachta Indica leaves. International Journal of Green Pharmacy. 10(4):200-203.







