Walid Abu Rayyan1 , Sami A. G. Alshammari2, Abdulaziz M. F. AL-Sammary2, Mutab S. S. ALShammari2, Nisreen Seder4, Luay F. Abu-Qatouseh1
, Sami A. G. Alshammari2, Abdulaziz M. F. AL-Sammary2, Mutab S. S. ALShammari2, Nisreen Seder4, Luay F. Abu-Qatouseh1 , Muna Bostami1, Kenza Mansoor3
, Muna Bostami1, Kenza Mansoor3 , Mohammed F. Hamad5, Ibrahim AlMajali6 and Wael Abu Dayyih3
, Mohammed F. Hamad5, Ibrahim AlMajali6 and Wael Abu Dayyih3
1Department of Pharmacology and Biomedical Sciences, Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan.
2College of Medicine, University of Hail, Hail, KSA.
3Department of Pharmaceutical Medicinal Chemistry and Pharmacognosy, Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan.
4School of Biomedical Science, Faculty of Health Sciences, University Sultan Zainal Abidin, 21300 Kuala Nerus, Terengganu, Malaysia.
5Department of Basic Sciences, College of Science and Health Professions, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
6Department of Medical Laboratory Sciences, Faculty of Science, Mutah University. Mutah, Jordan.
Corresponding Author E-mail: wabudayyih@uop.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/1547
Abstract
The increment in numbers of antimicrobial resistant strains along with the scarcity of new targets for drug industry has forced scientists to investigate deeply in the natural resources for new compounds with antimicrobial activity. Pergularia tomentosa is a member of the Apocynaceae family found in a wide geographical region including the Gulf region, Africa, and the Middle East. It is used as a remedy for the treatment of skin sores, asthma, and bronchitis. Dried plants of Pergularia tomentosa were subjected to extraction by using a Soxhlet extractor process to obtain essential oil and characterized by HPLC- Mass Spectroscopy (GC-MS). The essential oil was evaluated for antibacterial activity against pathogenic microorganisms by well diffusion method and confirmed by microdilution method. Additionally, we measured the antioxidant activity of the extracts using DPPH reagent. Phytochemical analysis has revealed variation in compositions and concentrations of P. tomentosa constituents grown in Hail from other agricultural regions. The lowest MIC was recorded with ethyl acetate extract MIC of 6.25 mg/ml against S. typhi, whereas, the ethanolic extract had the broadest effective against the five strains with a MIC of 25 mg/ml. In conclusion, we summarize a variation between the phytochemical constituents of P. tomentosa plants grown in the district of Hail and other geographical regions. In addition, there are several natural phytocompounds with an antimicrobial activity could be a good target for the antimicrobial and antioxidants industry.
Keywords
Antimicrobial; Antioxidants; HPLC-MS; Pergularia Tomentosa; Phytochemical
Download this article as:| Copy the following to cite this article: Rayyan W. A, Alshammari S. A. G, AL-Sammary A. M. F, AL-Shammari M. S. S, Seder N, Qatoosh L. F. A, Bostami M, Mansoor K, Hamad M. F, Al-Majali I. S, Daiyyah W. A. The Phytochemical Analysis and Antimicrobial Activity of Pergularia Tomentosa in North East Kingdom of Saudi Arabia KSA. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Rayyan W. A, Alshammari S. A. G, AL-Sammary A. M. F, AL-Shammari M. S. S, Seder N, Qatoosh L. F. A, Bostami M, Mansoor K, Hamad M. F, Al-Majali I. S, Daiyyah W. A. The Phytochemical Analysis and Antimicrobial Activity of Pergularia Tomentosa in North East Kingdom of Saudi Arabia KSA. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=23959 |
Introduction
The emergence of superbug strains between healthcare workers has obliged the scientific society to investigate deeper in the natural products for alternative compounds with antimicrobial activity.1,2 According to a report by the Infectious Diseases Society of America, around 70% of new cases administered to the hospitals in the US are involved with strains that show a potential no susceptibility to at least one drug.3,4 In the UK, nowadays, methicillin-resistant Staphylococcus aureus (MRSA) is considered a real concern in infection control accounting for around more than 50% of all S. aureus isolates whereas it had a low value a decade ago.2
Since the beginning of mankind, there is a wide use of herbal plants in the folk medicine due to their therapeutic and pharmaceutical properties.5-8 Pergularia tomentosa is a member of the Apocynaceae family (subfamily: Asclepiadeae) a yearly green plant with a distinctive odor, known in Hail city as “Aloonah’’.9,10 The plant is a climbing to semi erect perennial herb of around 30 cm, the stem is pale green-white in color, highly branched and usually grow vertically and milky latex is extracted from the plant.11
This plant is found in a wide geographical region including Gulf region (Saudi Arabia and Oman), Africa (north Sudan, Egypt, Ethiopia, Algeria, Niger and Kenya) and Middle East (Jordan, Iraq, Iran, Pakistan, and Afghanistan,).5, 12-14 Pergularia tomentosa is used as a remedy for the treatment of rheumatic fever, asthma, bronchitis, helminthiases and skin sores as cutaneous leishmaniosis.15,16 Furthermore, several publications have reported the cytotoxic, antioxidant and antibacterial activity of P. tomentosa.17-19 Up to date, this is the first study conducted on P. tomentosa in the region of Hail north east KSA that is focusing on the analysis of phytochemical components and elucidating the antioxidant and antimicrobial activity of this perennial.
Materials and Methods
Sampling
This study was performed in the northeast region of KSA at Hail district. Pergularia tomentosa (Fig. 1) was collected from the surrounding regions of Naqbeen village (a mountainous area) about 25 km from Hail.
 |
Figure 1: Pergularia tomentosa.
|
Leaves stem, and roots of P. tomentosa were collected locally in March 2017 in Hail, Southeast of KSA. Samples were collected from the waterways and slope in hilly areas and mixed together. The authenticity of the plant was confirmed by Dr. Mohammed Ahmed, and voucher specimens are maintained at the University of Hail (UOH) herbarium.
Preparation of Plant Extracts
The dust-free leaves stem and roots of Pergularia tomentosa were shade dried for five days and finely grounded. 30 grams of dried powder were mixed in equal amounts from each part and extracted with 200 ml of ethanol, chloroform, and ethyl acetate, consecutively, using Soxhlet extractor in order to separate phytochemical compounds based on their polarities. Filter paper Whatman No.1 was used to filter the crude extracts to remove impurities and debris and then the yield was concentrated by applying a vacuum at 35°C using a rotary evaporator. The concentrated extracts were subsequently dried aseptically using Lyophilization. Millipore distilled water was used to constitute inappropriate volume to obtain a final concentration of 200 mg/ml solution.
Phytochemical Studies
For choosing the best solvent system (gradient of solvents (A and B)), we set up the method according to the TLC and analytical HPLC/UV. The concentration of the extract for the LC-MS analysis was 1 mg/2 ML.20
Table 1: Chromatographic conditions of the HPLC MS.
| HPLC
Conditions |
Pump Flow Rate | Auto-sampler Injection Volume | Auto-sampler Temperature | Column Oven | |||||
| 1.00mL/minute | 25.00µL | 10.0∘C | 30.0∘C | ||||||
| Chromatography | Mobile Phase | Mixture of (60% ACN, +40% (675µL Triethylamine /1L of mixture,), pH adjusted to 7.1 with phosphoric acid | |||||||
| Column Type | Sepax GP-C18, (150 × 4.6 mm, 5 µm) | ||||||||
| Expected Retention Time | As seen in Table 230 nm | As seen in Table230 nm | |||||||
| MRM-Detection Conditions | Anlytes | Q1 | Q3 | Dwell | FP | DP | EP | CE | CXP |
| Category1 | 86 | 68.5 | 150 | 75 | 80 | 10 | 20 | 25 | |
| Category2 | 440 | 265 | 150 | 75 | 80 | 10 | 20 | 25 | |
| Mass-Spectra Conditions | CUR | CAD | IS | TEM | NEB | ||||
| 10 | 6 | 5500 | 400 | 5 | |||||
Ethanol layer of the extract of P. tomentose was subjected to qualitative analysis to determine the phytochemical composition for the plant. Liquid Chromatography-Mass Spectrometry (UHPLC system) with an autosampler and Waters nano Acquity HSS T3, 1.8 μm, 100 μm × 100 mm column was used in the analysis. H2O 0.1 % formic acid (A) (v/v, pH=2.17) and 90 % acetonitrile in H2O 0.1 % formic acid (B) was used as mobile phases at a flow rate of 0.4 ml/min. 1μm injection volume was used, the gradient elution for the injection was 5 % B during 0–2.5 min, a linear increase from 5 to 25 % B during 2.5–20 min, from 25 to 40 % B during 20–40 min and from 40 to 50 % B during 40–50 min, finally from 50 to 95 % B during 50–65 min followed by 15 min of maintenance. For identification of the eluent, we used Thermo Electron LTQ-Orbitrap XL mass spectrometer equipped with a nanoelectrospray ion source (ThermoFisher Scientific, Bremen, Germany) and operated under Xcalibur 2.1 version software, in positive ionization mode for the MS analysis using data-dependent automatic switching between MS and MS/MS acquisition modes (Table 1).12
Determination of Antioxidant Activity
Antioxidant properties of P. tomentosa extracts was determined in terms of scavenging free radical using DPPH method.21-23
The reduction in the concentration of 2,2-diphenyl-1-Picrylhydrazyl (DPPH) radicals by the activity of the antioxidants in P. tomentosa was measured and quantified by a colorimetric method. 0.02 g of DPPH reagent was dissolved in 1 L methanol; 3.9 mL of the solution is added to 0.1 mL of each sample that was diluted in a pure solvent of extraction at different concentrations. The mixtures were incubated for 90 minutes in a dark place at room temperature and the absorbance for each sample was measured at 517 nm using a UV/VIS spectrophotometer in triplicates.
The activity of free radical scavenging was disclosed as IC50 (µg/mL). The following equation was used to calculate the reduction in the amount of free DPPH radical by the extract:
DPPH scavenging effect (%) = ((A0-A1)/A0)*100
A0 is the control absorbance at 90 min, and A1 is the sample absorbance at 90 min. All samples were analyzed in triplicate.
Antimicrobial activity
We have conducted a preliminary study using the anti-bacterial susceptibility test to determine the minimal inhibitory concentrations (MICs) for the three herbal extracts (ethyl acetate, ethanol, and chloroform) against gram-positive, gram-negative and fungal strains. The protocol was conducted as defined in guidelines of National Committee for Clinical Laboratory Standard (NCCLS).24 Further, we compared their activities to reference compounds, fluconazole, and ciprofloxacin. We have determined MICs for a strain collection consisting of Staphylococcus aureus ATCC® 35556, Staphylococcus epidermidis ATCC® 12228, Escherichia coli ATCC® 25404, Salmonella typhi ATCC® 700931 and Candida albicans SC 5314. We covered a range of 6.25-100 mg/mL for the herbal extract against the strains. Bacterial strains were propagated on nutrient plates at 37°C for 24 hours. Whereas, we propagated C. albicans strains on sabouraud dextrose (Sab) plates at 25°C for 24 hours. Row number 1 on each microdilution plate was used as viability control of microbial cells. Rows 2 to 12 contained a decreasing amount of the herbal extract in a 1:1 serial dilution scheme resulting in a range of final concentrations from 6.5 mg/mL to 100 mg/mL. Ciprofloxacin was used as a reference drug for the antibacterial activity of the extracts whereas Fluconazole was used for the comparison of antifungal activity. TECAN Microtiter plate reader was used for obtaining the optical density at 450 nm for the microtiter plates after incubation at 37°C for 22 ± 2 hours, results were analyzed with Magellan software.
Results
Phytochemical Compounds
Characterization of the ethanol fraction of P. tomentosa was carried out using mass spectrometry. We identified 18 compounds in the aqueous extract; 14 compounds belonged to phenols and flavonoids whereas the last four had cardenolides structures. The results of the identified compounds were shown in Table 3. We also identified 3 flavonoids (Quercetin 3O-galactoside (8), Kaempferol 3-O-glucoside (11) and Kaempferol 3-O-malonylhexoside (14)) and one cardenolides (17) with LCMS techniques. This had also been isolated before in P. tomentosa [ref] and three other cardenolides (5, 17 and 18) which were not reported in this plant. Most of the identified flavonoids were belong to the flavone and flavonol 25,26 (Table 2, Fig. 2).
Table 2: Phytochemical components identified in the ethanolic extract of P. tomentosa.
| Identified Compounds in negative ion mode | Molecular formula | Antimicrobial activity | RT (min) | References | |
| 1 | Phenolic glycosid: Scrophenoside D | C28H34O17 | No | 4.12 | Li et al, 2014 |
| 2 | Phenolic: Synapoyl hexoside | C17H22O10 | No | 4.7 | Orqueda et al, 2017 |
| 3 | Flavone: Luteolin-di C-lucoside | C27H30O16 | Yes | 5.12 | Singh et al, 2015 |
| 4 | Phenolic: Feruloyl Glucoside | C16H20O9 | No | 9.1 | Frig et al, 2016 |
| 5 | Cardenolide: antiaroside G | C29H42O12 | No | 10.6 | Shi et al, 2013 |
| 6 | Flavonole: Kaempferol-malonyl-dihexosid | C30H32O19 | Yes | 12 | Salced et al, 2016 |
| 7 | Flavonole: Kampferol + glucose | C27H28O17 | Yes | 12.5 | Heneidak et al, 2006 |
| 8 | Flavonole: Quercetin 3-0-galactoside | C21H20O12 | Yes | 13.5 | Valente et al, 2016 |
| 9 | Flavonole: Isorhamnetin-3-0-glucoside | C22H22O12 | No | 13.6 | Haijuan et al, 2013 |
| 10 | Flavonole: Kaempferol 3-0-glucoside | C21H20O11 | Yes | 13.9 | Hettwer, 2016 |
| 11 | Phenolic: Ferulylmalic acid | C14H14O8 | No | 14.4 | Song et al, 2016 |
| 12 | Phenolic: glucuronic acid | C17H14O10 | No | 14.5 | Heneidak et al, 2006 |
| 13 | Flavonole: Kaempferol 3-0-malonylhexoside | C24H22O14 | No | 15.3 | Dugo et al, 2009 |
| 14 | Flavonole: Kaempferol-3-0-6 -acetyl-b-Dglucopyranoside | C23H22O12 | Yes | 15.7 | Ojwang, 2012 |
| 15 | Flavonole: quercetin-3-(6″-succinoyl)-glucoside | C25H24O15 | Yes | 16.3 | Hamed et al, 2006; |
| 16 | Cardenolide: Hydroxycalactin | C29H40O10 | No | 16.9 | Piacente et al, 2009 |
| 17 | Cardenolide: Antiaroside E | C29H42O10 | No | 18 | Shi et al, 2010 |
| 18 | Cardenolide: Antiaroside F | C35H52O15 | No | 18.5 | Shi et al, 2010 |
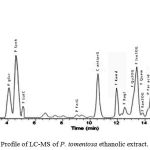 |
Figure 2: Profile of LC-MS of P. tomentosa ethanolic extract.
|
The names are according to the identified compounds in Table 2
The DPPH free Radical Scavenging Activity
The property of P. tomentosa extracts to scavenge free radicals was measured using a UV-visible spectrophotometer. Samples with plant extracts were analyzed covering a concentration range of 0.10‑2.0 mg/mL. In the same conditions, Butylhydroxytoluene (BHT) was also measured as a reference compound for the radical scavenging activity. The proportion of DPPH free radicals scavenging for both tested extracts and the positive control BHT are depicted in Table 3. The antioxidants properties of the extracts were expressed by the IC50 values, showing the sample concentration required to reduce fifty percent of DPPH free radicals (Table 3).
Linear regression analysis of the dose-response curve was used to determine the sample concentration required to reduce DPPH radical by 50% (IC50 value). The values are the mean of three determinations ± standard error.
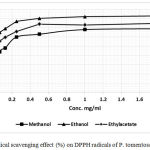 |
Figure 3: Radical scavenging effect (%) on DPPH radicals of P. tomentosa organs.
|
Table 3: DPPH radical scavenging activity expressed as IC50 values (μg/mL) of various extracts from P. tomentose.
| Extract | IC50 |
| Ethanol | 0.63 (0.993)* |
| Methanol | 0.58 (0.98) |
| Ethylacetate | 0.54 (0.981) |
| Butylhydroxytoluene (BHT) | 0.61(0.99) |
*R2: Correlation coefficient
Antimicrobial Activity
The three fractions of P. tomentosa showed different antimicrobial activity against the study strains; ethanol layer showed a broad inhibition activity against the five strains (S. aureus, S. epidermidis, E. coli, S. typhi and C. albicans) whereas ethyl acetate fraction had the most effective MIC values against only three strains (S. epidermidis, S. typhi, and C. albicans) as depicted in Fig (4). The lowest MIC value was 6.25 mg/mL for the collection strains. A summary of the MIC distribution for the three fractions against the test strains is given in Table 4. At the level of antibacterial activity, ethyl acetate fraction has the most effective value (MIC 6.25 mg/mL). It was evident from the MIC results that the lipophilicity and the low solubility profile have affected the activity of the prepared compounds and consequently their antimicrobial effect.
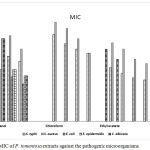 |
Figure 4: MIC of P. tomentosa extracts against the pathogenic microorganisms.
|
Table 4: MIC of Pergularia tomentosa extracts against pathogenic microorganisms.
| Conc. mg/ml | S. typhi | S. aureus | E. coli | S. epidermidis | C. albicans | |||||
|
Ethanol |
100 | 37.5 | 45 | 25 | 40 | 28 | ||||
| 50 | 31.5 | 41.5 | 21.5 | 25 | 26 | |||||
| 25 | 29 | 35 | 18 | 22 | 19 | |||||
| 12.5 | – | – | – | – | – | |||||
| 6.25 | – | – | – | – | – | |||||
|
Chloroform |
100 | 42 | 48 | – | – | – | ||||
| 50 | 37.5 | 45 | – | – | – | |||||
| 25 | 35 | 40 | – | – | – | |||||
| 12.5 | 33 | 33 | – | – | – | |||||
| 6.25 | – | – | – | – | ||||||
|
Ethyl acetate |
100 | 26 | 38 | – | – | 35 | ||||
| 50 | 23 | 35 | – | – | 30 | |||||
| 25 | 21 | 30 | – | – | – | |||||
| 12.5 | 20 | 28 | – | – | – | |||||
| 6.25 | 18 | 20 | – | – | – | |||||
|
Ciprofloxacin |
8 µg/ml | 25 | 28 | 24 | 30 | – | ||||
| 4 µg/ml | 20 | 23 | 19 | 20 | – | |||||
| 2 µg/ml | 15 | 14 | 13 | 18 | – | |||||
|
Fluconazole |
10 µg/ml | – | – | – | – | 17 | ||||
| 5 µg/ml | – | – | – | – | 13 | |||||
Discussion
This study has investigated the phytochemical composition and the biological properties of Pergularia tomentosa grown in the district of Hail. The results of metabolite profiling showed that there are 18 compounds (phenolics and cardenolides) in the extract. Fourteen compounds belonging to the phenolics and flavonoids, and 4 cardenolides were identified from leaves’ aqueous extract of P. tomentosa. Given that P. tomentosa is in Asclepiadaceae, we expected that it would have contained many cardenolides, but 79% of the identified compounds are phenolic, and 21% belong to the cardenolides. Other reports which investigated the aerial parts of this plant 9 demonstrated that its aerial parts are a rich source of flavonoids. Almost all studies showed that the roots of P. tomentosa are cardenolide-bearing part.6,9 We identified 4 cardenolides in leaves of this plant. These results suggested that the leaves of P. tomentosa are a rich source of flavonoids and could be a suitable source for valuable cardenolides. For example, one of the identified cardenolides is Ghalakinoside, which has a potent effect on cancer cells 27. The other three identified cardenolides (Antiaroside E-G), from the results of Shi et al (2010), exhibited strong cardiotonic activity, with a potent inhibitor of Na+/K+-ATPase. In addition, its phenolic-compounds can play a strong antioxidant role.8,28 In this field, Yakubu et al (2015) and Al Jabri (2013) confirmed the antioxidant effect of the extract of this plant.2,10,11,18
The antimicrobial activity for ethanol extract was the broadest whereas the lowest MIC was 6.25 mg/mL for ethyl acetate but surprisingly the inhibition was noticed in all strains the gram-positive, gram-negative and fungus. This points to inhibitory effect applied on a metabolic pathway or a process in gene transcription especially that the collection of pathogenic microorganism has different constituents in the cell membrane and ribosomal subunits.8,23,29-31
Additionally, phytochemical analysis has revealed variation in components and concentrations of P. tomentosa constituents grown at different agricultural regions.32 Interestingly, we can enumerate two important sources of antioxidants in P. tomentosa, firstly, the high concentrations of phenolic compounds in P. tomentosa which is considered to be a good source of powerful antioxidants, secondly, the hydroxyl groups in flavonoids which have the capability to react with DPPH radical by hydrogen atom donation to free radicals,7,33 while a highly positive correlation between total phenolic content and antioxidant activity was established in case of many plant species. 7,23
Conclusions
In conclusion, we summarize a variation between the phytochemical constituents of P. tomentosa plants grown in the district of Hail and other geographical regions. In addition, there are several natural phytocompounds with an antimicrobial activity could be a good target for the antimicrobial and antioxidants industry.
Conflict of Interest
There is no conflict of interest.
References
- Mahmoudi S., Khali M., Benkhaled A., Benamirouche K and Baiti I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pacific Journal of Tropical Biomedicine. 2016;6:239-245.
CrossRef - ADCOCK H. Pharmageddon is it too late to tackle growing resistance to anti-infectives? Pharmaceutical journal. 2002;269:599-600
- Alexandriaand V . I D S o America. Statement of the DSA Concerning Bioshield II: Responding to an Everchanging Threat. Arlington, VA: Society of America, IDSA. 2004.
- Mothana R. A., Kriegisch S., Harms M., Wende K and Lindequist U. Assessment of selected Yemeni medicinal plants for their in vitro antimicrobial anticancer and antioxidant activities. Pharmaceutical Biology. 2011;49:200-210.
CrossRef - Babaamer Z., Sekhri L., Hala A. J., Mahmoud A and ZARGA M. A. Extraction and identification of triterpenoids from Pergularia tomentosa L. 2013.
- Althunibat O. Y., Qaralleh H., Al-Dalin S. Y. A., Abboud M., Khleifat K.,Majali I. S., Aldal’in H. K., Rayyan W. A and Jaafraa A. Effect of Thymol and Carvacrol, the Major Components of Thymus capitatus on the Growth of Pseudomonas aeruginosa. Journal of Pure and Applied Microbiology. 2016;10:367-374.
- Mallah E. M., Rayyan W. S., Dayyih W. A., Elhajji F. D., Mansour K. A., Al-Majali I. S and Arafat T. A. Dose-Dependent Synergistic effect of Pomegranate Juice on the Bioavailability of Sildenafil in Rats by Using HPLC Method. Lat Am J Pharm. 2016;35:1277-1284.
- Majali I. S., Oran S. A., Khaled M. k., Qaralleh H., Rayyan W. A and Althunibat O. Y. Assessment of the antibacterial effects of Moringa peregrina extracts. African Journal of microbiology research. 2015;9:2410-2414.
CrossRef - Heneidak S., Grayer R. J., Kite G. C and Simmonds M. S. Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochemical systematics and ecology. 2006;34:575-584.
CrossRef - Akroum S., Bendjeddou D., Satta D and Lalaoui K. Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia. American-Eurasian Journal of Scientific Research. 2009;4:93-96.
- Al Jabri S. A. H. Chemical and Bio-analytical Studies on Pergularia tomentosa and Species from the Mentha Genus, University of Leicester. 2013.
- Erfanzadeh R., Shahbazian R and Zali H. Role of plant patches in preserving flora from the soil seed bank in an overgrazed high-mountain habitat in northern Iran. 2014.
- Ahmed M. M. E. O ., Essam G. A. S., Said M. E., Niwa M. Cardenolides and βSitosterol Glucoside from Pergularia tomentosa. Natural Product Sciences. 2000;6:142-146.
- Al-Said M. S., Hifnawy M. S., McPhail A. T and McPhail D. R. Ghalakinoside a cytotoxic cardiac glycoside from Pergularia tomentosa. Phytochemistry. 1988;27:3245-3250.
CrossRef - Hamed A. I., Plaza A., Balestrieri M, L., Mahalel U. A ., Springuel I. V., Oleszek W., Pizza C and Piacente S. Cardenolide glycosides from Pergularia tomentosa and their proapoptotic activity in Kaposi’s sarcoma cells. Journal of natural products. 2006;69:1319-1322.
CrossRef - Green P. W., Veitch N. C., Stevenson P. C and Simmonds M. S. Cardenolides from Gomphocarpus sinaicus and Pergularia tomentosa (Apocynaceae Asclepiadoideae) deter the feeding of Spodoptera littoralis. Arthropod-Plant Interactions. 2011;5:219.
CrossRef - S Piacente M. M., Nève N. D., Dewelle J., Hamed A., Kiss R and Mijatovic T. Cardenolides from Pergularia tomentosa display cytotoxic activity resulting from their potent inhibition of Na K-ATPase. Journal of natural products. 2009;72:1087-1091.
CrossRef - Yakubu R., Musa F., Lukman A and Sheikh F. Activity guided fractionation with Antimicrobial Evaluation of Pergularia Tomentosa L.(Asclepiadacea) whole plant. British Microbiology Research Journal. 2015;8:567-576.
CrossRef - Goyder D. A revision of the genus Pergularia L.(Apocynaceae Asclepiadoideae). Kew Bulletin. 2006;245-256.
- D’Urso G., Maldini M., Pintore G., d’Aquino L., Montoro P and Pizza C. Characterisation of Fragaria vesca fruit from Italy following a metabolomics approach through integrated mass spectrometry techniques. LWT-Food Science and Technology. 2016;74:387-395.
CrossRef - Brand-Williams W., Cuvelier M. E and Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology. 1995;28:25-30.
CrossRef - Hosseinzadeh H.,Zarei H and Taghiabadi E. Antinociceptive, anti-inflammatory and acute toxicity effects of Juglans regia L. leaves in mice. Iranian Red Crescent Medical Journal. 2011;13:27.
- Al-Nadaf A. H., Seder N. J and Rayyan W. A. Wound healing; antimicrobial and anti-oxidant activity for Jordanian Juglans Regia L. unripe fruits. Journal of Innovations in Pharmaceutical and Biological Sciences. 2018;5:26-34.
- P Wayne. National committee for clinical laboratory standards. Performance standards for antimicrobial disc susceptibility testing, 12:01-53 (2002).
- Mari A., Lyon D.,Fragner L., Montoro P., Piacente S., Wienkoop S., Egelhofer V., and Weckwerth W. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics. 2013;9:599-607.
CrossRef - Li P., Su W., Xie C., Zeng X ., Peng W and Liu M. Rapid Identification and Simultaneous Quantification of Multiple Constituents in Nao-Shuan-Tong Capsule by Ultra-Fast Liquid Chromatography Diode-Array Detector Quadrupole Time-of-Flight Tandem Mass Spectrometry. Journal of chromatographic science. 2014;53:886-897.
CrossRef - Shi L. S., Kuo S. C., Sun H. D., Morris-Natschke S. L., Lee K. H and Wu T. S . Cytotoxic cardiac glycosides and coumarins from Antiaris toxicaria. Bioorganic & medicinal chemistry. 2014;22:1889-1898 .
CrossRef - Shi L. S., Liao Y. R., Su M. J., Lee A. S.,Kuo P. C ., Damu A. G., Kuo S. C., Sun H. D., Lee K. H and Wu T. S. Cardiac glycosides from Antiaris toxicaria with potent cardiotonic activity. Journal of natural products. 2010;73:1214-1222.
CrossRef - Burger-Kentischer A., Finkelmeier D.,Keller P., Bauer J., Eickhoff H.,Kleymann G., Rayyan W. A., Singh A., Schroppel K., Lemuth K., Wiesmuller K. H and Rupp S. A screening assay based on host-pathogen interaction models identifies a set of novel antifungal benzimidazole derivatives. Antimicrobial agents and chemotherapy. 2011;55:4789-4801.
CrossRef - Sweidan K., Engelmann J., Abu W. R., Sabbah D.,Abu M. Z., Al-Qirim T., Al-Hiari Y., Abu G. S and Shattat G. Synthesis and preliminary biological evaluation of new heterocyclic carboxamide models. Letters in Drug Design & Discovery. 2015;12:417-429
CrossRef - Sweidan K ., Abu W. R., Abu M. Z., El-Abadelah M. M and Mohammad H. A. Y. Synthesis and Antibacterial Evaluation of Model Fluoroquinolone-Benzylidene Barbiturate Hybrids. Letters in Organic Chemistry. 2014;11:422-425.
CrossRef - Cushnieand T. T., Lamb A. J. Antimicrobial activity of flavonoids. International journal of antimicrobial agents. 2005;26:343-356.
CrossRef - Lahmar I.,Belghith H., Ben F. A and Belghith K. Nutritional composition and phytochemical, antioxidative and antifungal activities of Pergularia tomentosa L. BioMed research international. 2017.
CrossRef







