Manuscript accepted on :14-Nov-2018
Published online on: 27-11-2018
Plagiarism Check: Yes
Reviewed by: Ian Martins
Second Review by: Dr. Amarendranath Choudhury
Eun-Jin Yang1 , Sungchan Jang2, Kwang Hee Hyun3
, Sungchan Jang2, Kwang Hee Hyun3 , Eun-Young Jung4
, Eun-Young Jung4 , Seung-Young Kim2
, Seung-Young Kim2 and Chang-Gu Hyun1
and Chang-Gu Hyun1
1Department of Chemistry and Cosmetics, Jeju National University, Jeju 63243, Korea.
2Department of Pharmaceutical Engineering and Biotechnology, Sunmoon University, Chungnam 31460, Korea.
3Helios Co., Ltd., Sanchundan Dong-gil 16, Jeju 63243, Korea.
4Department of Beauty Art, Chejuhalla University, 38 Halladaehak-ro, Jeju 63092, Korea.
Corresponding Author E-mail: cghyun@jejunu.ac.kr
DOI : https://dx.doi.org/10.13005/bpj/1546
Abstract
The anti-inflammatory activity and non-toxicity of Sonchus oleraceus extract (J6) were tested by measuring its effect on the levels of nitric oxide (NO), prostaglandin E2 (PGE2), and the pro-inflammatory cytokines, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. We treated the RAW264.7 cells with various concentrations (50, 100, or 200 μg/mL) of J6. Our results showed that J6 inhibited the production of NO, PGE2, and pro-inflammatory cytokines in a concentration-dependent manner, without compromising cell viability. In addition, we provided supporting evidence that the inhibitory activity of J6 on the production of NO and PGE2 occurred via the downregulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively. Our findings suggest that J6 is a new source for anti-inflammatory drugs and ingredients for healthcare products that include functional cosmetics.
Keywords
Anti-inflammation; COX-2; iNOS; PGE2; Sonchus oleraceus; TNF-α
Download this article as:| Copy the following to cite this article: Yang E. J, Jang S, Hyun K. E, Jung E. Y, Kim S. Y, Hyun C. G. Anti-Inflammatory Activity of Sonchus oleraceus Extract in Lipopolysaccharide-Stimulated RAW264.7 Cells. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Yang E. J, Jang S, Hyun K. E, Jung E. Y, Kim S. Y, Hyun C. G. Anti-Inflammatory Activity of Sonchus oleraceus Extract in Lipopolysaccharide-Stimulated RAW264.7 Cells. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24319 |
Introduction
Lipopolysaccharide (LPS), a well-known endotoxin, is found in the outer membrane of Gram-negative bacteria. LPS induces the expression of inflammatory mediators in macrophages, such as RAW 264.7 cells, and monocytes1 and increases the production of nitric oxide (NO) and cytokines in the early stage of inflammation.2
Nitric oxide synthase (NOS), which mediates inflammation, exists as three isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). eNOS is mainly expressed in endothelial cells and nNOS expression is restricted mostly to the nervous system and pancreatic beta cells. These two isoenzymes are constitutively expressed and play a key role in homeostasis, including vasodilation, blood flow control, and nerve signal transmission.3 iNOS, in contrast, is induced by LPS and enzymatically generates the pro-inflammatory mediator, NO.4,5 Another inflammatory enzyme, cyclooxygenase (COX), exists as two isoforms, namely COX-1 and COX-2. COX-1 is expressed in almost every tissue and is involved in homeostasis and protection of body organs. COX-2 is mostly involved in inflammatory responses6,7 and its expression is induced by LPS and inflammatory cytokines.8 The expression of iNOS and COX-2, their production of NO and prostaglandin E2 (PGE2), respectively, and the generation of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, are common markers of inflammation9.
Sonchus oleraceus is a biennial, conical-shaped plant that belongs to the Asteraceae family. It can grow up to 1 m high and has been utilized as a medicinal compound and food source for livestock and humans. Its seedling leaves can be eaten uncooked. This plant contains a high content of flavonoid compounds, such as kaempferol, luteolin. quercetin, quercimeritrin, and chrysanthemin.10–13
Previous studies mainly investigated the polyphenol content and antioxidant activity of S. oleraceus when grown at different locations, with seasonal changes, and after exposure to various environmental factors.10–13 However, there has been little study on the pharmacological and functional properties of S. oleraceus and its use as an ingredient in functional cosmetics. Some widely used synthetic materials such as oxybenzone, mineral oil, and parabens can reportedly cause adverse effects such as liver damage, abnormal body growth, and gastrointestinal bleeding. 20 As a result, interest in biotic materials with minimal side effects is increasing. Thus, the aim of the present study was to investigate the inhibitory activity of J6 S. oleraceus extract on the expression of inflammatory mediators in LPS-stimulated macrophages and to explore its potential as a biotic material.
Materials and Methods
J6 Procurement and Cell Culture
J6 was obtained from the Jeju Biodiversity Institute.19 The murine macrophage cell line RAW264.7 was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 100 units/mL penicillin-streptomycin and 10% (v/v) fetal bovine serum (FBS) at 37°C and 5% CO2.
Cytotoxicity Assay
An MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was implemented to assess the cytotoxicity of the J6 extract. RAW264.7 cells were plated at 1.8×105 cells/200uL/well in a 96-well plate and incubated for 18 h prior to the 24 h-treatment of 1 µg/mL LPS and different concentrations of J6 extract (50, 100, and 200 µg/mL) at 37°C and 5% CO2. Fifty microliters of MTT reagent was then added and incubated for 4 h. The spent culture medium was completely removed and 200 µL dimethyl sulfoxide (DMSO, Sigma, MO, USA) was added to dissolve the formazan precipitate. The absorbance at 540 nm was then measured using a microplate reader (Bio-Tek Instrument Inc., Vermont, WI, USA). The average absorbance for each sample group was used to evaluate cell viability.
NO Assay
RAW264.7 cells were plated at 1.8×105 cells/200uL/well in a 24-well plate and incubated for 18 h prior to the 24 h-treatment of 1 µg/mL LPS and different concentrations of J6 extract (50, 100, and 200 µg/mL) at 37°C and 5% CO2. One hundred microliters of culture medium from each well and an equal volume of Griess reagent were mixed and incubated at room temperature for 10 min15 followed by the measurement of absorbance at 540 nm using an enzyme-linked immunosorbent assay (ELISA) reader. The Griess reagent (1% (w/v) sulfanilamide, 0.1% (w/v) N-(1-naphthyl)ethylenediamine in 2.5% (v/v) phosphoric acid) reacts with nitrite in the culture medium to estimate the amount of NO produced. Values were calculated by using a standard curve of sodium nitrite (NaNO2).
PGE2, IL-1β, IL-6, and TNF-α ELISAs
RAW264.7 cells were cultured as described above and treated for 24 h with 450 µL of 1 µg/mL LPS and 50 µL of 10-times-concentrated 1 mg/mL J6 extract. The culture medium was then centrifuged at 12,000 rpm for 3 min and the resulting supernatant was used to measure the content of PGE2, IL-1β, IL-6, and TNF-α. All samples were kept at −20°C until quantification. PGE2 and the other three pro-inflammatory cytokines were quantified using specific ELISA kits (R&D Systems Inc., Minneapolis, MN, USA); the R-squared values for the standard curves were ≥ 0.99.
Western Blot Analysis
As previously described, RAW264.7 cells were cultured and treated with LPS and J6 extract. After washing twice in PBS, proteins were isolated in lysis buffer [1X RIPA buffer (Upstate Cell Signaling Solution, Lake Placid, NY, USA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM Na3VO4, 1 mM NaF, 1 μg/mL aprotinin, 1 μg/mL pepstatin, and 1 μg / mL leupeptin] for 1 h, followed by centrifugation. The protein-containing supernatant was subjected to 10% SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) at 200 mA for 2 h. The resulting membrane was blocked at room temperature with 5% skim milk in 0.05% Tween-20/Tris-buffered saline (T/TBS) followed by incubation with the primary antibody at 4°C overnight. The primary antibodies were iNOS antibody (1:5000, Calbiochem, USA), COX-2 antibody (1:1000, BD Biosciences Pharmingen, USA), and β-actin antibody clone AC-74 (1:10,000, Sigma, USA). After washing four times in T/TBS, the membrane was incubated with 5,000- or 10,000-fold diluted secondary antibody (Jackson ImmunoResearch, East Grove, PA, USA), followed by washing thrice in T/TBS. The proteins of interest were detected and quantified using an ECL kit and an imaging densitometer (Model GS-700; Bio-Rad, Hercules, CA, USA).
Statistical Analysis
All data are expressed as means ± standard deviations (SDs). Statistical differences between samples were resolved by Student’s t-tests.
Results and Discussion
Effect of J6 on Cell Viability
For the cytotoxicity assay, murine RAW 264.7 macrophage cells were treated for 24 h with 1 µg/mL LPS alone or in combination with various concentrations of J6 extract (50, 100, or 200 µg/mL). An increase in cell viability and no toxicity were observed in the J6-treated samples relative to that of the control group (Fig. 1, line plot), which was consistent with the results of a previous study on the anti-inflammatory effect of Scutellariae radix extract.16 Up to 200 µg/mL of J6 extract, near the cytotoxic threshold concentration, was used further to assay the inhibitory activity of J6 on the generation of NO, PGE2, and pro-inflammatory cytokines.
Effect of J6 on NO Generation
The effect of J6 extract on the generation of NO, a critical mediator of the inflammatory response, was examined (Fig. 1, bar graph). RAW264.7 cells were treated with LPS and J6 extract as described above and the generated NO was quantified as nitrite using a Griess assay. Relative to that of the LPS-only-treated group, NO production was decreased by 64% at the peak concentration of 200 µg/mL J6 extract. This confirmed the potent inhibitory activity of J6 on NO production and its potential to inhibit iNOS expression.
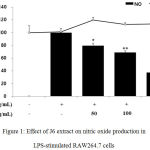 |
Figure 1: Effect of J6 extract on nitric oxide production in LPS-stimulated RAW264.7 cells.
|
Cells were stimulated with 1 μg/mL LPS alone or in combination with various concentrations (50, 100, or 200 μg/mL) of J6 for 24 h. Nitric oxide production was determined by the Griess reagent method. Cell viability was determined using an MTT assay from the 24-h culture of cells stimulated with LPS (1 μg/mL) in the presence of J6. All data are expressed as the means ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone. IC50= 158.6 μg/mL.
Effect of J6 extract on PGE2 production in LPS-stimulated RAW264.7 cells
RAW 264.7 cells were plated and treated with LPS and J6 extract as described in the NO assay and PGE2 generation was then assessed using a PGE2 ELISA kit. The cells were treated with J6 at various concentrations of 50, 100, and 200 µg/mL. Relative to that of the cells treated with LPS only, PGE2 production was substantially decreased over the J6 concentration range of 50–200 µg/mL; this reduction reached 95.5% at the peak concentration of 200 µg/mL J6 (Fig. 2). Therefore, J6 extract inhibited LPS-induced PGE2 production concentration-dependently.
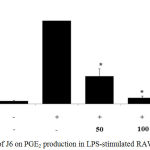 |
Figure 2: Effect of J6 on PGE2 production in LPS-stimulated RAW264.7 cells.
|
Cells were stimulated with 1 μg/mL LPS with or without various concentrations (50, 100, or 200 μg/mL) of J6 for 24 h. PGE2 production was measured by ELISA. The data represent the means ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone. IC50=18.3 μg/mL.
Effect of J6 Extract on Pro-inflammatory Cytokine (IL-1β, IL-6, and TNF-α) Generation
Cytokines act as mediators in the activation, proliferation, and differentiation of immune cells and typical pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are well recognized as modulators of inflammatory responses both in vitro and in vivo.17 The effect of J6 extract on the production of these three pro-inflammatory cytokines in the presence and absence of LPS was examined. The secretion of IL-1β, IL-6, and TNF-α was increased in the medium of LPS-treated cells (Fig. 3, 4, 5). Relative to that of the LPS-only-treated group, IL-1β and IL-6 production were concentration-dependently decreased by J6 extract, reaching 43.5% (Fig. 3) and 80% (Fig. 4) reduction, respectively, at 200 µg/mL J6. Similarly, TNF-α production was decreased in a concentration-dependent manner (Fig. 5). This confirmed the anti-inflammatory effect of J6 extract on secretion of the three pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α, in LPS-stimulated RAW264.7 cells.
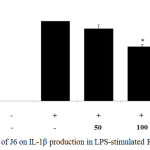 |
Figure 3: Effect of J6 on IL-1β production in LPS-stimulated RAW264.7 cells.
|
Cells were stimulated with 1 μg/mL of LPS only or with various concentrations (50, 100, and 200 μg/mL) of J6 for 24 h. IL-1β production was measured by ELISA. The data represent the means ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone.
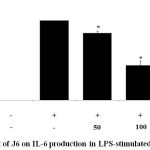 |
Figure 4: Effect of J6 on IL-6 production in LPS-stimulated RAW264.7 cells.
|
Cells were stimulated with 1 μg/mL of LPS with or without various concentrations (50, 100, and 200 μg/mL) of J6 for 24 h. IL-6 production was measured by ELISA. The data represent the means ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone. IC50=114.5 μg/mL.
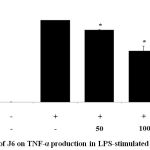 |
Figure 5: Effect of J6 on TNF-α production in LPS-stimulated RAW264.7 cells.
|
Cells were stimulated with 1 μg/mL LPS only or with various concentrations (50, 100, or 200 μg/mL) of J6 for 24 h. TNF-α production was measured by ELISA. The data represent the means ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone.
Effect of J6 on Protein Expression of iNOS and COX-2
iNOS and COX-2 are known as key players in the synthesis of NO and PGE2 during inflammation.18 Based on our findings that J6 inhibits the production of NO and PGE2 in a concentration-dependent manner, we used western blotting to examine whether the reduced production of NO and PGE2 was associated with reduced protein expression of iNOS and COX-2. Our results demonstrate that J6 extract had a suppressive effect on the LPS-induced expression of iNOS and COX-2 in a concentration-dependent manner (Fig. 6, 7). This also showed that J6 inhibits NO and PGE2 generation via downregulation of iNOS and COX-2 expression, respectively.
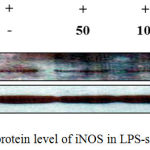 |
Figure 6: Effect of J6 on protein level of iNOS in LPS-stimulated RAW 264.7 cells.
|
RAW 264.7 cells (6.0 × 105 cell/mL) were stimulated with LPS (1 µg/mL) in the presence of J6 (50, 100, or 200 μg/mL) for 24 h. Whole-cell lysates (30 µg) were prepared and subjected to 10% SDS-PAGE; the expression level of iNOS and β-actin were determined by western blotting. β-Actin was used as a loading control. Representative images are shown.
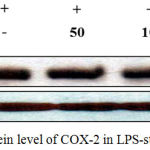 |
Figure 7: Effect of J6 on protein level of COX-2 in LPS-stimulated RAW 264.7 cells.
|
RAW 264.7 cells (6.0 × 105 cell/mL) were stimulated with LPS (1 µg/mL) in the presence of J6 (50, 100, or 200 μg/mL) for 24 h. Whole-cell lysates (30 µg) were prepared and subjected to 10% SDS-PAGE; the expression level of COX-2 and β-actin were determined by western blotting. β-actin was used as a loading control. Representative images are shown.
Conclusions
S. oleraceus has been valued for animal and human consumption but there have been few studies on its pharmacological activity and toxicity. Our study confirmed that J6 extract was not toxic and exerted an inhibitory effect on the production of NO, PGE2, and pro-inflammatory cytokines in LPS-stimulated macrophages. We found that during LPS-stimulation J6 extract inhibited the generation of NO and PGE2 in a concentration-dependent manner. We also examined whether J6 was involved in modulating the production of pro-inflammatory cytokines and found it to concentration-dependently inhibit the secretion of three pro-inflammatory mediators (i.e., IL-1β, IL-6, and TNF-α), suggesting that it has potent anti-inflammatory effects. Moreover, the protein level of both iNOS and COX-2 was drastically decreased with increasing concentrations of J6 extract. Further research is required to clarify that the down-regulation of iNOS and COX-2 expression is through the downregulation of NF-κB activation and MAPK phosphorylation.21,22 These results provide strong support that J6 is a potential new source for anti-inflammatory drugs and ingredients for healthcare products, including functional cosmetics.
Acknowledgements
This work was funded by the 2017 Jeju Industry-academic Converged Zone (MOTIE, 14150240).
References
- Park S. J., Shin J. S., Cho W., Cho Y. W., Ahn E.M., Baek N. I., Lee T. K. Inhibition of LPS induced iNOS, COX-2 and cytokines expression by kaempferol-3-o-β-D-sophoroside though the NF-kB inactivation in RAW264.7 cells. Kor J Pharmacogn. 2008;39:95-103.
- Higuchi M., Hisgahi N., Taki H., Osawa T. Cytolytic mechanisms of activated macrophages, tumor necrosis factor and L-arginine-dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J Immunol. 199;144:1425-1431.
- Moncada S., Higgs E. A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995;9:1319-1330.
CrossRef - Guzik T. J., Korbut R., Adamek-guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469-487.
- Barnes P. J., Liew F. Y. Nitric oxide and asthmatic inflammation. Immunol Today. 1995;16:128-130.
CrossRef - Dubois R. N., Abramson S. B., Crofford L., Gupta R. A., Simon L. S., De Putte L. B.V., Lipsky P. E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063-1073.
CrossRef - Smith W. L., Michael G. R., De-Witt D. L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157-33160.
CrossRef - Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol. 1998;38:97-120.
CrossRef - Yun H. J., Heo S. K., Yi H. S., Kim C. H., Kim B. W., Park S. D. Anti-inflammatory effect of injiho-tang in RAW264.7 cells. Korean J Herbology. 2008;23:169-178.
- Howard L. R., Pandjaitan N., Morelock T., Gil M. I. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J Agric Food Chem. 2002;50:5891-5896.
CrossRef - Ma M., Hong C. L., An S. Q., Li B. Seasonal, spatial, and inter specific variation in quercetin in apocynum venetum and poacynum hendersonii, Chinese traditional herbal teas. J Agric Food Chem. 2003;51:2390-2393.
CrossRef - Guil-Guerrero J. L., Gimnez-Gimnez A., Rodriguez-Garcia I., Torija-Isasa M. E. Nutritional composition of Sonchus species (A. asper L, S. oleraceus L and S. tenerrimus L). J Sci Food Agric. 1998;76:628-632.
CrossRef - Yin J., Kwon G. J., Wang M. H. The antioxidant and cytotoxic activities of Sonchus oleraceus L. extracts. Nutr Res Pract. 2007;3:189-194.
CrossRef - Yang E. J., Hyun J. M., Lee N. H., Hyun C. G. In vitro screening of Korean halophytes for cosmeceutical ingredients. Int J ChemTech Res. 2016;9:541-547.
- Delgado-Pertinez M., Gomez-Cabrera A., Garrido A. Predicting the nutritive value of the olive leaf (olea europea): digestibility and chemical composition and in vitro studies. Anim Feed Sci Technol. 2000;87:187-201.
CrossRef - Yoon S. B., Han H. S., Lee Y. J. Effect of Scutellariae radix extract on the proinflammatory mediators in RAW 264.7 cells induced by LPS. Korean J Herbology. 2011;26:75-81.
- Kong S. M., Jang S. A., Sohn E. H., Bak J. P., Sohn E. S., Koo H. J., Yoon W. J., Kwon J. E., Jeong Y. J., Meng X., Han H. S., Kang S. C. Comparative study of Litsea japonica leaf and fruit extract on the anti-inflammatory effects. Korean J Plant Res. 2015;28:145-152.
CrossRef - Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639-653.
CrossRef - Eun-Jin Y., Mi Hyun J., Lee N. H and Chang-Gu H. In vitro screening of Korean halophytes for cosmeceutical ingredients. International Journal of ChemTech Research. 2016;9(8):541-547.
- Lourie B.,Smith R., Toxout T. Getting Harmful Chemicals Out of Our Bodies and Our World. St. Martin’s Griffin; Reprint edition. 2015.
- Uto T., Suangkaew N., Morinaga O., Kariyazono H., Oiso S and Shoyama Y. Eriobotryae Folium Extract Suppresses LPS-Induced iNOS and COX-2 Expression by Inhibition of NF-kB and MAPK Activation in Murine Macrophages. The American Journal of Chinese Medicine. 2010;38(05):985-994.
CrossRef - Kim H. D., Chung H. J., Yoon S. J., Ha M. Y., Bae S., Lee K. E., Jung J. K., Kim S. M., . Kim J. Y., Kim K. M and Chung Y. H. Ginsenoside Rd Inhibits the Expression of iNOS and COX-2 by Suppressing NF-kB in LPS-Stimulated RAW264.7 cells and mouse liver. J. Ginseng Res. 34(1):42-63.







