Manuscript accepted on :13-Sep-2018
Published online on: 17-09-2018
Plagiarism Check: Yes
Reviewed by: Priya Ponmudi
Second Review by: Ayan Chatterjee
Final Approval by: Dr. Javad Sharifi-Rad
Nabila A. El-Laithy1, Elsayed M. E. Mahdy2, Eman R. Youness1, Nermeen Shafee3, Mohamed S.S. Mowafy4 and Mahmoud M. Mabrouk1
1Department of Medical Biochemistry, National Research Centre, Cairo, Egypt.
2Department of Chemistry , Faculty of Science, Helwan University, Helwan, Egypt.
3Department of Pathology, National Research Centre, Cairo, Egypt.
4Critical care unit, Faculty of Medicine, Cairo University, Cairo, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1483
Abstract
Our was to determine the impact of CoenzymeQ10 (Co Q10) and vitamin C alone or in combination on oxidative stress in brain tissue of rats during endotoxemia induced by single intraperitoneal dose of Lipopolysaccharide (LPS), 500µg/kg. Both CoQ10&vitamin C were given orally to rats with doses (200&100 mg/kg) respectively for 7successive days prior induction of endotoxemia .LPS injected, with Co Q10 with doses (100 &200 mg/kg) &vit. C (50&100 mg/kg).In addition CoQ10 and vitamin C together in doses (100&50 mg/kg) & (200&100 mg/kg) respectively were added to LPS-treated rats. Then euthanized 4 hours later. Histopathological assessment of brain tissue was done. Results: LPS injection induced oxidative stress in brain tissue, resulting in marked increase in malondiadehyde (MDA), nitrite (NO) and Amyloid beta (Aβ), while decreasing reduced glutathione (GSH), paraoxonase-1 (PON1) and brain derived neurotrophic factor (BDNF).CoQ10 and vit.C administration with doses(200&100 mg/ kg) before endotoxemia result in reduction of brain MDA, NO and Aβ, while increasing levels of GSH, PON1 and BDNF compared to controls. The addition of both Co Q10 &vit.C to LPS- treated rats lead to decrease of brain NO, MDA and Aβ, also increase of GSH, PON1 and BDNF. This effect was more obviouswith high doses, this due to the ameliorating effect of both CoQ10 and vit.C on oxidative stress of brain tissue during endotoxemia.This consisted with the histopathological results. Conclusion: this work focuses on the possible role of CoQ10 &vit.C as antioxidants in protecting brain tissue.
Keywords
Co Q10-vitamin C-LPS-oxidative stress-Aβ-BDNF
Download this article as:| Copy the following to cite this article: El-Laithy N. A, Mahdy E. M. E, Youness E. R, Shafee N, Mowafy M. S. S, Mabrouk M. M. Effect of Co Enzyme Q10 Alone or in Combination With Vitamin C on Lipopolysaccharide-Induced Brain Injury in Rats. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: El-Laithy N. A, Mahdy E. M. E, Youness E. R, Shafee N, Mowafy M. S. S, Mabrouk M. M. Effect of Co Enzyme Q10 Alone or in Combination With Vitamin C on Lipopolysaccharide-Induced Brain Injury in Rats. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22604 |
Introduction
The imbalance between the reactive oxygen species generation (ROS) and the mechanism responsible for elimination of the ROS causes oxidative stress in tissues andcells.1 Common ROS has many kinds like, hydroxyl radical, hydrogen peroxideandsuperoxideanion radical.Also, superoxide and high levels of nitric oxide give increase in peroxynitrite, which can perform both oxidation and nitrative stresstogether with similar reactive nitrogen species (RNS). Oxidative stress has the ability to damage all cellular macromolecules, including protein side chains,sugars, nucleic acids& polyunsaturated lipids, thus plays a vital role in the pathogenesis of many neurological diseases.
Coenzyme Q10 (CoQ10) or Ubiquinone is fat-soluble, vitamin-like substance present in nearly all eukaryotic cells, mainly in mitochondria.It is a component of the electron transport chain that contributes to aerobic cellular respiration, which produces energy in the form of ATP.2In the last few years, there has been many researches which focused on its antioxidant properties in several cellular compartments.3 CoQ10 has shown defending effect against neuronal loss in several animal models of neurodegeneration and the capacity of CoQ10 to limit disease progression in clinical trials put into consideration to explore neuroprotective activity in many of the neurodegenerative diseases.4
Ascorbic acid (vit.C) is a water-soluble micronutrient.It is a robust antioxidant which perform directly via scavenging of ROS and indirectly through reproducing of other antioxidant systems.5 Yaman et al., (2010) had proved that oxidative damage to macromolecules in vivo can be minimized by supplementation with large doses of this vitamin.This is important in the synthesis and stabilization of neurotransmitters.6
Brain derived neurotrophic factor (BDNF), plays a vital role in supporting neuronal survival & function during development in adulthood.7 Mitochondrial respiratory coupling at complex I is motivated by BDNF. It plays anessential role in brain metabolism and oxygen utilization.8 Bothglial cell line-derived growth factor and BDNF protect against H2O2 induced neuronal death in patients with idiopathic Parkinson’s disease.9 Accordingly, antioxidant effects in lesioned spinal cords of rats can be produced by giving of BDNF.10
Amyloid β (Aβ) has been shown play a viable role inAlzheimer’sdisease (AD) and, thus, may play a main role in the pathogenesis of the disease. Butterfield., et al (2013) has shown that Aβ induces protein oxidation and lipid peroxidation both in vitro and in vivo, The excessive production and accumulation of Aβ are responsible for the progression and pathogenesis of AD.11Hence, the use of treatments to decrease Aβ formation or the downstream oxidative stress connected with Aβ could be useful in preventing, curing, or delaying the progression of AD.12
Lipopolysaccharide (LPS) is the main component of the outer membrane of Gram-negative bacteria and is responsible for the initiation of the host response to the microorganisms. In response to LPS, macrophages secrete pro-inflammatory cytokines as the interleukins IL-6, IL-12,IL-1β&tumor necrosis factor(TNF)-αand reactive oxygen species (ROS) like superoxide anion.13
The currentwork was therefore designed to inspect the impact of oral supplementation of Co Q10 and ascorbic acid alone or in combination on brain oxidative stress in a model of endotoxemia.For this purpose, rats were injectedintraperitoneally with a subseptic dose of LPS.MDA, NO, GSH, PON1 as well as BDNF and Aβ were determined in brain homogenate.14
Materials and methods
The current study was done on sixty six adult albino Wistarratsweighing from 100 to 120gm,3 months old that are obtained from theanimal house colony of the National Research Centre (NRC),Egypt. The animals were maintained on standard diet andwater, housed in stainless steel cages in controlled temperature(23 ± 1oC). All animals were housed in animal house for a week prior to experiments.Also they received human care according to the guidelines foranimal experiments which were approved by the Ethical Committeeof Medical Research, National Research Centre (NRC).
Chemicals and drugs
Apurified and lyophilized E. coli endotoxin from(Serotype055: B5, Sigma, USA) was utilized and dissolvedin sterile saline, aliquoted, and frozenat−80°C.CoQ10 were purchased from (MEPACO-MEDIFOOD,Egypt). CoQ10 was prepared by dissolving in 1% tween80 as vehicle. Vitamin C (Sigma, MO, USA) was prepared by dissolving in distilled water.
Study Design
66 Female albino rats weighing 100-120 g will be divided into 11 groups (six rats in each). Rats were treated with tween80 (group 1).Animals treated with saline (group 2), LPS (500μg/kg, i.p.) (group3),Co Q10(200mg/kg)(group 4),Co Q10 at 100mg/kg + LPS (group 5), Co Q10 at 200mg/kg + LPS (group 6),Vitamin C (100mg/kg)(group 7),Vitamin C at 50mg/kg + LPS (group 8),Vitamin C at 100mg/kg + LPS (group 9),Co Q10 at 100mg/kg+Vitamin C at 50mg/kg + LPS (group 10)andCo Q10 at 200mg/kg+Vitamin C at 100mg/kg + LPS (group 11).After four hours,rats were euthanized after LPS injection.Brains were removed&washed with ice-cold saline solution (0.9 % NaCl) and stored at −80 °C for the biochemical analysis.
Lipid peroxidation Determination
The level OFlipid peroxidation in the brain tissue homogenates was determined by evaluatingmalondialdehyde level (MDA) according to Ruiz-Larreaet al.15 In this assay, MDA (along with its equivalents) reacts with thiobarbiturateproducing a red colored complex that read at 532 nm.
Determination of nitric oxide
Nitric oxide level in the supernatants of the brain tissue homogenate was determined indirectly by measuring the level of nitrite using the Griess reagent, according to Moshageet al.16
Determination of reduced glutathione
Reduced glutathionewas evaluated in supernatantof brain tissue rendering to Ellman (1959).17 This was based on the reduction of Ellman’s reagent by–SHgroups in GSHformingnitromercaptobenzoic acid. The color of this acid can be determined spectrophotometrically.
Paraoxonase 1 activitydetermination
The activity of paraoxonase 1 (PON1) in brain tissue homogenates was measured using substratephenyl acetate as described previously.18|Herein, PON1 catalyzes phenyl acetatecleavage, forming phenol. The rate of the formation of phenol which is measured spectrophotometrically at 270 nm.
Determination of Brain derived neurotrophic factor
BDNF was measured using ELISA kit (Glory Science Co., Ltd, Del Rio, TX, USA).
Determination of Brain Amyloid β
Amyloid β was measured ELISA kit (Glory Science Co., Ltd, Del Rio, TX, USA).
Histopathological studies
Specimens of brain were dissectedright away after death and fixed in 10% neutral-buffered formal saline,then dehydrated in ascending grades of alcohol (70% – 80% – 90% and finally absolute alcohol), cleared in xylene, soaked in soft paraffin wax at 55°C and entrenched in hard parrafin. Sections of 6 µm thick were cut and stained with Haematoxylin and eosin19 for histopathological investigation.
Statistical Test
Data weregiven as the mean ± SE from six rats in eachgroup. Significance of the statistics was assessed using ANOVA test followed by post hoc LSD test. Value of less than 0.05 was consideredstatistically significant.
Results
Brain Biochemical results
Impact of Coenzyme Q10 on oxidative stress
Malondialdehyde (MDA)
Co Q10 at a dose 200 mg/kgb/w revealed decrease in MDA level in brain by 20.9 % (p<0.05) in comparison with the control group. Following LPS, MDA in the brain tissue wassignificantly increased by 108.47 % (p<0.001) when compared with the tween80 control group. MDA showed decrease by 27.69 % (p<0.05) & 39.35 % (p<0.05) in rats given Co Q10 (100 mg/kg & 200 mg/kg) and LPS compared with the corresponding LPS control value (table1).
Nitrate (NO)
|Nitrate decreased by 14.96 % (p>0.05) after Coenzyme Q10 injection at a dose 200 mg/kg in comparison with the tween80 control group. In disparity, marked and significant elevation of nitric oxide by 59.09% (p<0.05) following injectionof LPS. Rats treated with Co Q10 and LPS, nitrates decreased in brain in dose 100mg/kgbody weight by 28.73% ( p<0.05 ) and in dose 200 mg/kgb/w by 35.28% (p<0.05) if compared with LPS group(table1).
Reduced glutathione (GSH)
Coenzyme Q10resulted in increase in GSH by 31.15% (p<0.05) if compared with the tween80 control group. Following LPS, GSH in brain was significantly decrease by 23.96% (p<0.05). When Coenzyme Q10 given to LPS –treated rats result in increasing by 62.92%(p<0.05) and 65.73% (p<0.05) in rats taken Co Q10 (100 mg/kg &200 mg/kg b/w) and LPS compared with the corresponding LPS control value(table1).
Impact of Coenzyme Q10 on paraoxonase activity (PON1)
The administration of only Co Q10 result in increase in PON1 activity by 19.09 % (p <0.05) in comparison with the corresponding tween80 control value. Following LPS, PON1 in the brain was significantly decreased by 46.27 % (p <0.05) compared with the tween80 control group. PON1 showed increase by 55.42 % (p<0.05) & 74.69 % (p<0.05) in rats given Co Q10 (100 mg/kg & 200 mg/kg b/w) and LPS when compared with the corresponding LPS control value (table1).
Impact of Coenzyme Q10 on derived neurotrophic factor (BDNF)
Coenzyme Q10 resulted in increase in BDNF by 38.45% (p<0.05) when compared with the tween80 group. Following LPS, BDNF decrease by 30.78% (p<0.05), Coenzyme Q10 when given to rats treated with LPS, resulted in an increase by 16.89% (p<0.05) and 26.53% (p<0.05) in rats given Co Q10 (100 mg/kg & 200 mg/kg b/w) and LPS when compared with the corresponding LPS control value(table 1).
Effect of Coenzyme Q10 on Amyloid β (Aβ)
The administration of only Co Q10 at a dose 200 mg/kg body weight resulted in decrease in the level of Aβ in brain by 17.50 % (p<0.05) if compared with the tween80 control group. Following LPS, Aβ in the brain was significantly increased by 149.25 % (p<0.05) when compared with the tween80 group. Aβ showed decrease by 37.29 % (p<0.05) & 48.17 % (p<0.05) in rats given Co Q10 (100 mg/kg & 200 mg/kg b/w) and LPS when compared with the corresponding control value of LPS (table 1).
Effect of Vitamin C on brain oxidative stress
Malondialdehyde (MDA)
Vitamin C only at a dose 100 mg/kg b/w cause decrease in MDA level in brain by 12.08 % (p>0.05) in comparison with the control group. Following lipopolysaccharid, MDA level in brain was significantly augmented by 120.09 % (p<0.05) as compared with the control group. MDA showed decrease near 19.58 % (p<0.05) and 30.33 % (p<0.05) in rats given the vitamin (50 mg/kg & 100 mg/kg b/w) and LPS compared with the corresponding LPS value (table 2).
Nitric oxide (NO)
In brain, nitric oxide decreased by 5.86 % (p>0.05) after vitamin C injection at a dose 100 mg/kg b/w in comparing with the tween80 group. In contrast, marked & significant elevation of nitric oxide by 64.34% ((p<0.05) following LPS injection. In rats taken vitamin C and LPS , nitric oxide decrease in dose 50mg/kg b/w by 15.04% ( p<0.05 ) and in dose 100 mg/kg b/w by 22.65% (p<0.05) as compared with LPS group(table 2).
Reduced glutathione (GSH)
Vitamin C resulted in increase in GSH by 16.66% (p<0.05) in comparison with the control group. Following LPS, GSH showed obvious and significant decrease by 25.83% (p<0.05). Vitamin C when added to LPS treated rats result in increasing by 26.56% (p<0.05) and49.70 %(p<0.05)in rats given vitamin C (50 mg/kg &100 mg/kg b/w) and LPS as compared with the corresponding LPS value(table 2).
Impact of Vitamin C paraoxonase activity (PON1) on brain
Administration of vitamin C only result in increase in paraoxonase activity by 5.67 % (p >0.05) as compared with the corresponding control value. Following LPS, PON1 in the brain was significantly diminished by 47.63 % (p <0.05) in comparison with the control group. PON1 showed increase by 24.09 % (p<0.05) and 37.95 % (p<0.05) in rats given Vitamin C (50 mg/kg & 100 mg/kg b/w) and LPS as compared with the corresponding LPS control value (table 2).
Impact of Vitamin C on Brain derived neurotrophic factor (BDNF)
Vitamin C resulted in increase in BDNF by 11.54% (p>0.05) as compared with the control group. Following LPS, BDNF showed obvious and significant decrease by 34.97% (p<0.05). Vitamin C when given to LPS –treated rats result in increasing by 7.10% (p>0.05) and12.59% (p>0.05)in rats taken vitamin C (50 mg/kg &100 mg/kg b/w) and LPS compared with the corresponding LPS control value(table2).
Effect of Vitamin C on Amyloid β (Aβ)
The administration of vitamin C alone in a dose 100 mg/kg b/w cause decrease of the level of Aβ in brain by 8.81 % (p<0.05) as compared with saline control group. Following LPS, Aβ in the brain was significantly increased by 152.82 % (p<0.001) as compared with the control group. Aβ showed decrease by 34.35 % (p<0.05) and 36.51 % (p<0.05) in rats given Vitamin C (50 mg/kg & 100 mg/kg b/w) and LPS compared with the corresponding LPS control value (table2).
Impact of both Coenzyme Q10 & Vitamin C with different concentrations in addition to LPS on malondialdehyde (MDA)
The group treated with Coenzyme Q10 (100 mg/kg b/w), vitamin C (50 mg/kg b/w) and LPS showed decrease in MDA by 43.36%(p<0.05) and the group treated with Coenzyme Q10 (200 mg/kg b/w), vitamin C (100 mg/kg b/w) and LPS showed decrease in MDA by 48.44%(p<0.05) compared with the corresponding LPS control value (table 3).
Effect of both Coenzyme Q10 and Vitamin C with different concentrations in addition to LPS on NO
In brain, nitric oxide lowered by 34.35 % (p<0.05) in the group treated with Coenzyme Q10 (100 mg/kg b/w), vitamin C (50 mg/kg b/w) and LPS, decreased by 34.35 % (p<0.05) in the group treated with Coenzyme Q10 (100 mg/kg b/w), vitamin C (50 mg/kg b/w) and LPS, as compared with LPS group (table 3).
Impact of both Coenzyme Q10 and Vitamin C with different concentrations in addition to LPS on Reduced glutathione (GSH)
The group treated with Coenzyme Q10 (100 mg/kg body weight), vitamin C (50 mg/kg b/w) and LPS showed noticeable and significant increase of GSH by 80.43%(p<0.001) and the group treated with Coenzyme Q10 (200 mg/kg b/w), vitamin C (100 mg/kg b/w) and LPS showed marked and significant increase in GSH by 86.71%(p<0.001) as compared with the corresponding LPS control value (table 3).
Impact of both Coenzyme Q10 and Vitamin C with different concentrations in addition to LPS on brain paraoxonase activity (PON1)
The group treated with Coenzyme Q10 (100 mg/kg b/w), vitamin C (50 mg/kg b/w) and LPS showed marked & significant increase in paraoxonase by 146.98%(p<0.001) and the group treated with Coenzyme Q10 (200 mg/kg b/w), vitamin C (100 mg/kg b/w) and LPS showed noticeable and significant increase in PON1 by 183.13%(p<0.001) as compared with the corresponding LPS control value (table 3).
Effect of both Coenzyme Q10 and Vitamin C with different concentrations in addition to LPS on Brain derived neurotrophic factor (BDNF)
The group treated with vitamin C (50 mg/kg b/w), Coenzyme Q10 (100 mg/kg b/w) and LPS showed marked and significant increase in BDNF by 36.73%(p<0.05) and the group treated with Coenzyme Q10 (200 mg/kg b/w), vitamin C (100 mg/kg b/w) and LPS showed marked and significant increase in BDNF by 45.18%(p<0.05) as compared with the corresponding LPS control value (table3).
Impact of both Coenzyme Q10 and Vitamin C with different concentrations in addition to LPS on Amyloid β (Aβ)
In brain tissue, Aβ decreased by 58.04 % (p<0.05) in the group treated by Coenzyme Q10 (100 mg/kg b/w), vitamin C (50 mg/kg b/w) and LPS, and Aβ decreased by 59.3 % (p<0.05) in the group treated by Co Q10 (100 mg/kg b/w vitamin C (50 mg/kg b/w) and LPS, and compared with LPS group (table3).
Table 1: Effect of Coenzyme Q10 (Co Q10), LPS or both on malondialdehyde (MDA), nitric oxide (NO), reduced glutathione (GSH), paraoxonase 1 activity (PON1),Brain derived neurotrophic factor (BDNF) and Amyloid β(Aβ) in rat brain.
| Parameters
Groups |
MDA
(nmol /g. tissue) |
NO
(µmol/g. tissue) |
GSH
(µmol/g. tissue) |
PON1
(kU/l) |
BDNF
(pg/ml) |
Aβ
(pg/ml) |
| Tween80 | 32.58±1.04 | 29.75±1.28 | 3.98±0.06 | 3.09±0.06 | 50.81±0.71 | 217.85±1.32 |
| CoQ10(200 mg/kg) | 25.77±0.92* | 25.30±0.80 | 5.22±0.04* | 3.68±0.08* | 70.35±0.82* | 179.72±1.87* |
| LPS | 67.92±2.49* | 47.33±0.83* | 3.03±0.05* | 1.66±0.05* | 35.17±4.69* | 543.01±3.49* |
| CoQ10(100 mg/kg )+LPS | 49.11±1.86# | 33.73±1.00# | 4.93±0.03# | 2.58±0.05# | 41.11±0.62# | 340.49±1.75# |
| CoQ10(200 mg/kg)+LPS | 41.19±1.41# | 30.63±0.03# | 5.02±0.06# | 2.90±0.06# | 44.50±0.96# | 281.41±1.16# |
Results are presented as mean±SE, P < 0 .05 = statistically significant.
*P < 0 .05 versus Tween80 control
#P<0.05 versus LPS group
Table 2: Impact of LPS, vitamin C or together on (MDA), (NO), (GSH) , (PON1), (BDNF) and (Aβ) in rat brain.
| Parameters
Groups |
MDA
(nmol/g. tissue) |
NO
(µmol/g. tissue) |
GSH
(µmol/g. tissue) |
PON1
(kU/l) |
BDNF
(pg/ml) |
Aβ
(pg/ml) |
| Saline | 30.86±1.82 | 28.80±1.19 | 4.08±0.06 | 3.17±0.07 | 54.09±0.85 | 214.78±1.52 |
| Vit.C (100 mg/kg) | 27.13±0.75 | 27.11±1.04 | 4.76±0.03* | 3.35±0.07 | 60.33±1.16 | 195.84±1.38* |
| LPS | 67.92±2.49* | 47.33±0.83* | 3.03±0.05* | 1.66±0.05* | 35.17±4.69* | 543.01±3.49* |
| Vit.C (50 mg/kg )+LPS | 54.62±2.35# | 40.21±0.04# | 3.83±0.05# | 2.06±0.07# | 37.66±0.78 | 356.44±2.39# |
| Vit.C (100 mg/kg)+LPS | 47.32±1.49# | 36.61±1.35# | 4.57±0.06# | 2.29±0.06# | 39.6±0.57 | 344.72±1.82# |
Results are presented as mean± SE, P < 0 .05 considered statistically significant.
*P < 0 .05 versus saline control
#P<0.05 versus LPS control group
Table 3: Impact of both Coenzyme Q10 and Vitamin C with different concentrations in addition to LPS on (MDA), nitric oxide (NO), (GSH), paraoxonase 1 activity (PON1),Brain derived neurotrophic factor (BDNF) and Amyloid β (Aβ) in rat brain.
| Parameters Groups | MDA
(nmol /g. tissue) |
NO
(µmol/g. tissue) |
GSH
(µmol/g. tissue) |
PON1
(kU/l) |
BDNF
(pg/ml) |
Aβ
(pg/ml) |
| Saline | 30.86±1.82 | 28.80±1.19 | 4.08±0.06 | 3.17±0.07 | 54.09±0.85 | 214.78±1.52 |
| Tween80 | 32.58±1.04 | 29.75±1.28 | 3.98±0.06 | 3.09±0.06 | 50.81±0.71 | 217.85±1.32 |
| LPS | 67.92±2.49* | 47.33±0.83* | 3.03±0.05* | 1.66±0.05* | 35.17±4.69* | 543.01±3.49* |
| CoQ10(100mg/kg)
+Vit.C(50mg/kg)+LPS |
38.47±1.49# | 31.07±0.33# | 5.46±0.05# | 4.10±0.07# | 48.09±0.9# | 227.85±1.59# |
| CoQ10(200mg/kg)
+Vit.C(100mg/kg)+LPS |
35.02±1.15# | 30.46±0.07# | 5.65±0.06# | 4.70±0.06# | 51.06±0.61# | 221.09±0.87# |
Results are presented as mean± SE, P < 0 .05 considered statistically significant.
*P < 0 .05 versus saline control
#P<0.05 versus LPS control group
Brain Histopathological results
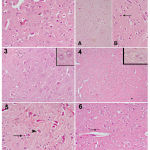 |
Figure 1
|
a photomicrograph of a cerebral cortex tissue section from:
1) control vehicle rat shows normal shaped neurons. 2) control +ve (LPS) shows a large number of small dark neurons that appear in the higher magnification part with acidophilic cytoplasm and deeply stained nuclei (arrow). 3) a control – ve rat, 4) control co φ10 – 200 mg rat shows quite normal structure of cerebral cortex. 5) rat (received LPS + 100 mg co φ10 ) shows a noticeable reduction in dark neurons number, some are still small in size (arrow), while others began to regain their normal size (arrowhead). 6) rat (received LPS + 200 mg co φ10 ) shows normalization of almost all the cells.
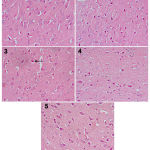 |
Figure 2
|
a photomicrograph of a cerebral cortex tissue section from:
1) a rat (control Vit. C) shows normal cerebral cortex tissue, 2) rat (received LPS + 50 mg Vit. C) shows slight decrease in small dark neurons, 3) rat (received LPS + 100 mg Vit. C) shows only a few cells with signs of degeneration (arrow). 4) rat (received LPS + 100 mg co φ10 + 50 mg Vit. C) shows some neurons with signs of degeneration are still observed. 5) rat (received LPS + 200 mg co φ10 + 100 mg Vit. C) shows normalization of cerebral cortex tissue.
Discussion
Herein in the present study, we investigated the impact of oral administration of Coenzyme Q10 (CoQ10), vitamin C and both together on brain oxidative stress due to intraperitoneal administration of lipopolysaccaride (LPS) in rat. Following injection of LPS at sub-septic dose of 500μg/kg, levels of reduced glutathione (GSH) in brain homogenate decreased along with the increased values of the lipid peroxidation (MDA). Glutathione tripeptide in brain (glycyl-glutamyl-cysteine) is the most plentiful cytosolic thiol and is the ultimate important antioxidant. Also it acts as a co-factor for glutathione transferase, glutathione peroxidase. Glutathione ca directly scavenge reactive oxygen species as singlet oxygen and hydroxyl radical. The ratio of oxidized and reduced types of glutathione determines the redox state of the cell.20,21 The depletion of GSH in brain reflects the consumption of the antioxidant as the augmented free radical generation by endotoxin . It was also found that elevated lipid peroxidation, depleted glutathione reductase & GSH activity occur after the peripheral injection of LPS.22,23
Lipopolysaccaride also elevated nitric oxide (NO) values in brain. Diminished concentrations of nitric oxide generated by endothelial (eNOS) or neuronal (nNOS) isoforms of nitric oxide synthase (NOS) are important in maintenance of vascularity and in neurotransmission.24 In contrast, a high levels of nitric oxide for long period may cause death of nerve cell. This is established in cases of inflammation, infection, ischemia and trauma as microglia is activated by reactive oxygen intermediates and inflammatory mediators. This stimulate the release of exaggerated amounts of nitric oxide through nitric oxide synthase (iNOS).25 Studies indicated that not only induction of eNOS mRNA but also iNOS in brain by LPS treatment.26 NO motivate neurotoxicity via suppression of neuronal respiration leading to glutamate release and then excitotoxicity.27 It inerreacts with the superoxide anion producing more reactive peroxynitrite anion (ONOO−), that is able to oxidizeproteins, lipids, and DNA.28
In our work, the bustle of paraoxonase was reduced in brain of LPS-treated rat. The (PON1) hydrolyzes the lethal metabolites of many organophosphorus pesticides. paraoxonase enzyme is formed mainly by liver cells and secreted to the plasma. It prevents oxidation of high density lipoproteins.29,30,31 Also PON1 conserves cellular membranes against lipid peroxidation.32 The decrease in its activity has been recommended to inhance the neurodegenerative diseases, such as, Parkinsonism.33
Our results demonstrated an inhibition of Brain-derived neurotrophic factor (BDNF) level, that acts on specific neurons of the peripheral and central nervous system.34 It was confirmed that the administration of LPS lowered BDNF mRNA expression levels in the hippocampus of rats.35 Also previous researches showed that BDNF can protect the cells against oxidative-mediated damage indicating that this factor may have antioxidant properties.36,37 Although Zhu et al., (2014) found no change in levels of BDNF expression following LPS administration.38
In our model, LPS results in an increase of brain Amyloid-β (Aβ),this in agreement with Zhu et al., (2014). Increased expression of Aβ in the hippocampus was spotted following the chronic administration of LPS. This indicating that the enhanced expression of Aβ may be a major factor in the pathogenesis of cognitive dysfunction.38
Oral pretreatment with CoQ10 for 7 days before endotoxemia produced significant brain protection as evidenced by reducing MDA content to the basal level, decreasing NO production, and Aβ .while there was significant increase of GSH,PON-1 and BDNF compared to control group .The neuroprotection observed in our model by CoQ10 may be refer to the fact that CoQ10 is found to be an essential component of the electron transport chain where it acts as an electron donor and acceptor. It has also been suggested that it could bridge a defect in the electron transport chain, improve membrane fluidity, and act as an antioxidant.39Matthews et al., (1998)showed that oral administration of CoQ10 increases its mitochondrial concentrations in the cerebral cortex and provided further evidence that CoQ10 can produceneuroprotective effects that might be valuable in the treatment of neurodegenerative diseases.40
In this study the effect of both CoQ10&vit.C administration to the LPS treated rats can alleviating the effect against LPS-induced oxidative stress in brain tissue. Concerning CoQ10 this in agreement with Song et al. (2017), they suggest the beneficial effect of CoQ10 on antioxidant defense system in laboratory animals under sever oxidative stress conditions.41 Also Kantar et al., (2005)reported the importance of vit.C as antioxidant for protection against degenerative process caused by oxidative stress.42
In this study, the impact of oral pretreatment of vitamin C, with dose of 100mg/kg for 7days on brain tissue result in inhibition of MAD &NO, at the same time increased the level of GSH, this agree with Halliwell et al., (1987).43 D’Uscio et al., (2003) said that vitamin C scavenges the ROS via very rapid electron transfer that inhibit lipid peroxidation.44 There was increase of PON-1 level which was agree with Zargari and Saeedy (2017).45 Also there was a decrease in Aβ that was in agreement with Kook et al., (2014). Vitamin C supplementation results in reduction of amyloid plaque deposition, BBB (blood brain barrier) disruptions and mitochondrial dysfunction in the brains of 5XFAD mice, an animal model for Alzheimer’s disease (AD).46 There was increase of BDNF concentration, although in study of Rai et al., (2013)vitamin C with dose 200mg/kg resulted in diminished BDNF expression in rats that have not received stress suggesting that vitamin C acted as pro-oxidant linking the connectivity between the oxidative damage and BDNF expression.47
The potential effect of CoQ10and vitamin C when adding together with doses of 200 and100 mg/kg respectively result in modulating oxidative stress in endotoxemic brain tissue generated by restrained stress may determine their clinical usefulness as supplemental nutritional therapeutic agent in disorders affecting the brain free radical metabolism .This was consistent with the result of histopathology, who found that these concentrations more effective than other(100 and 50mg/kg).As vitamin C and CoQ10 are reported to act as an effective antioxidants of major importance for protection against diseases and degenerative processes caused by oxidative stress.
The histopathological results proved that LPS caused damage to brain tissue. Such results are emphasized bypang et al., 2003 who reported that intracerebral delivery of lipopolysaccharide (LPS) preferentially induces white matter damage, loss of immunoreactivity of immature OL markers, hypomyelinationand increased size of lateralventricles.48
In conclusion, CoQ10 treatment alone or in combination with vitamin C may be beneficial in preventing endotoxin-induced oxidative damage inbrain, and therefore show potential for clinical use.
References
- Sayre L. M., Perry G and Smith M. A. Oxidative stress and neuro toxicity. Chem Res Toxicol. 2008;21:172–88.
CrossRef - Bhadri N., Sanji T.,Guggilla H. M and Razdan R. Amelioration of Behavioural, Biochemical and Neurophysio logical Deficits by Combination of Mono sodium Glut a mate with Resveratrol Alpha-Lipoic Acid Coenzy me Q 10 in Rat Model of Cisplatin-Induced Peripheral Neuropathy. The Scientific World Journal. 2013;8. Article ID 565813.
- Littarru G. P and Langsjoen P. Coenzyme Q 10 and statins biochemical and clinical implications. Mitochondrion. 2007;7:168–174.
CrossRef - Galpern W. R and Cudkowicz M. E . Coenzy me Q treatment of neurodegenerative diseases of aging. Mitochondrion. 2007;7:146–153.
CrossRef - Robichova S., Slamenova D., Chalupa I and Sebová L. DNA lesions and cytogenetic changes induced by N-nitrosomorpholine in Hep G2, V 79 and VH 10 cells the protective effects of Vitamins A, C and E. Mutat Res. 2004;560:91-99.
CrossRef - Yaman H., Çayci T., Seyrek M., Akgül E. Ö., Kurt G. Y., Aydin İ., Yaren H., Çakir E., Özcan Ö., Çimen B., Türközkan N and Erbil M. K. Effects of vitamin A and C and melatonin on 3-nitrotyrosine formation in guinea pig heart under lipopolysaccharide-induced stress. Turk J Med Sci. 2010;40(5):715-721.
- He T., Katusic Z. S. Brain-derived neurotrophic factor in creases expression of Mn SOD in human circulating angiogenic cells. Microvasc. Res. 2012;83(3):366-371.
CrossRef - Markham A., Cameron I., Franklin P and Spedding M . BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20:1189–96.
CrossRef - Onyango I. G., Tuttle J. B and Bennett J. P Jr. Brain-derived growth factor and glial cell line-derived growth factor use distinct intra cellular signalling pathways to protect PD cybrids from H2O2 induced neuronal death. Neurobiol Dis. 2005;20:141–54.
CrossRef - Gardiner J., Barton D., Overall R and Marc J. Neurotrophic Support and Oxi dative Stress Converging Effects in the Normal and Diseased Nervous System.The Neuroscientist. 2009;15(1).
CrossRef - Butterfield D. A and Boyd-Kimball D. A myloid β-Peptide(1-42) Contributes to the Oxi dative Stress and Neurodegeneration Found in Alzheimer Disease Brain. Brain Pathol. 2004;14:426-432.
CrossRef - Ganjei J. K. Targeting amyloid precursor protein secretases: Alzheimer’s disease and beyond. Drug News Perspect. 2010;23:573–584.
CrossRef - Gorąca A and Asłanowicz−Antkowiak K. Prophylaxis with α-lipoic acid against lipopolysaccharide-induced brain injury in rats. Arch. Immunol. Ther. Exp. 2009;57:141–146.
CrossRef - Quan N., Stern E. L., Whiteside M. B and Herkenham M. Induction of pro-inflammatory cytokine m RNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72–80.
CrossRef - Ruiz-Larrea M. B., Leal A. M., Liza M., Lacort M and de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver micro somes. Steroids. 1994; 59(6):383–8.
CrossRef - Moshage H., Kok B., Huizenga J. R and Jansen P. L. Nitrite and nitrate determinations in plasma a critical evaluation. Clin Chem. 1995;41:892–896.
- Ellman G. L . Tissue sulfhydryl groups. Arch Biochem. 1959;82:70–77.
CrossRef - Higashino K., Takahashi Y and Yamamura Y. Release of phenyl acetate esterase from liver microsomes by carbon tetrachloride. Clin. Chim. Acta. 1972;41:313–20.
CrossRef - Drury R.A.B and Wallington F. A. Corleton’s Histological Technique 4th Ed. Oxford, New York, Toronto Oxford university press. 1980.
- Halliwell B and Gutteridge J. M. C. Free radicals in biology and medicine, 3rd edn. Clarendon Oxford. 1999.
- Dickinson D. A and Forman H. J. Cellular glutathione and thiols metabolism. Biochem.Pharmacol. 2002;64:1019–1026.
CrossRef - Noble F., Rubira E., Boulanouar M., Palmier B., Plotkine M., Warnet J. M., Marchand-Leroux C and Massicot F. Acute systemic inflammation induces central mitochondrial damage and amnesic deficit in adult Swiss mice. Neurosci Lett. 2007;424:106–110.
CrossRef - Jacewicz M., Czapski G.A., Katkowska I and Strosznajder R. P. Systemic administration of lipopolysaccharide impairs glutathione redox state and object recognition in male mice.The effect of PARP-1 inhibitor. Folia Neuropathol. 2009;47:321–328.
- Thippeswamy T., McKay J. S., Quinn J. P and Morris R. Nitric oxide a biological double-faced janus—is this good or bad? Histol Histopathol. 2006;21:445–458.
- Pérez-Nievas B. G., Madrigal J. L., García-Bueno B et al. Corticoster one basal levels and vulnerability to LPS-induced neuro inflammation in the rat brain. Brain Res. 2010;1315:159–168.
CrossRef - Iwase K., Miyanaka K., Shimizu A., Nagasaki A., Gotoh T., Mori M. and Takiguchi M. Induction of endothelial nitric-oxide synthase in rat brain astrocytes by systemic lipopolysaccharide treatment. J Biol Chem. 2000;275:11929–11933.
CrossRef - Bal-Price A and Brown G. C. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J Neurochem. 2000;75:1455–1464.
CrossRef - Moncada S., Palmer R. M .J and Higgs E. A. Nitric oxide physiology, patho physiology and pharmacology. Pharmacol Rev. 1991;43:109–142.
- Du L. B. N. Human serum paraoxonase arylesterase. In Kalow W (ed) Pharma cogenetics of drug metabolism. Pergamon, Elmford. 1992;51–91.
- Primo-Parmo S. L., Sorenson R. C., Teiber J and Du L. B. N. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multi gene family. Genomics. 1996;33:498–507.
CrossRef - Mackness B., Quarck R., Verreth W., Mackness M. and Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in amouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550.
CrossRef - Rodrigo L., Hernández A. F., López-Caballero J. J., Gil F and Pla A. Immu no his to chemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. 2001.
- Hancock D. B., Martin E. R., Mayhew G. M., Stajich J. M., Jewett R., Stacy M. A., Scott B. L., Vance J. M., Scott W. K. Pesticide exposure and risk of Parkinson’s disease a family-based case-control study. BMC Neurol. 2008;8:6.
CrossRef - Yan Q., Rosenfeld R. D., Matheson C. R., et al. Expression of brain‑derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431‑448.
CrossRef - Lapchak P. A., Araujo D. M and Hefti F. Systemic interleukin‑1 beta decreases brain‑derived neurotrophic factor messenger RNA expression in the rat hippo campal formation. Neuroscience. 1993;53: 297‑301.
CrossRef - Mattson M. P., Maudsley S and Martin B. BDNF and 5-HT a dynamic duo in age-related neuronal plasticity and neurode generative disorders. Trends Neurosci. 2004;27:589–594.
CrossRef - Singh S., Ahmad R., Mathur D., Sagar R. K., Krishana B., Arora R and Sharma R. K. Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease. Indian J ExpBiol. 2006;44:699.
- Zhu B., Wang Z. G., Ding J., Liu N., Wang D. M., Ding L. C and Yang C. Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippo campus. Experimental and Therapeutic Medicine. 2014;7:750-754.
CrossRef - Frei B., Kim M.C and Ames B. N. Ubiquinol-10 is an effective lipidsoluble antioxidant at physiological concentrations. Proc Natl Acad Sci USA. 1990;87:4879–83.
CrossRef - Matthews R. T., Yang L., Browne S., Baik M and Beal M.F. Coenzy me Q 10 administration increases brain mitochondrial concentrations and exerts neuro protective effects. Proc Natl Acad Sci USA. 1998;95:8892–7.
CrossRef - Song M. H., Kim H. N., Lim Y and Jang I..S. Effects of coenzy me Q 10 on the antioxidant system in SD rats exposed to lipopolysaccharide-induced toxicity. Lab Anim Res. 2017;33(1):24-31.
CrossRef - Kanter M., Coskun O and Armutcu F. Protective effect of vitamin C,alone or in combination with vitamin A,on Endotoxin-Induced oxidative renal tissue damage in rats. Tohoku J. Exp.Med. 2005;206:155-162.
CrossRef - Halliwell B., Wasil M and Grootveld M. Biologically significant scavenging of the myeloperoxidase-derived oxidant hypo chlorous acid by a scorbic acid. FEBS Lett. 1987;213:15-17.
CrossRef - D’Uscio L.V., Milstien S., Richardson D., Smith L and Katusic Z. S. Long-Term Vitamin C Treatment Increases Vascular Tetrahydro biopter in Levels and Nitric Oxide Synthase Activity. 2003.
- Zargari F and Saeedy H. T . Effects of Vitamin C on Paraoxonase1 Arylesterase Activity in Rats Exposed to Arsenic. Iranian Journal of Toxicology. 2017;11:3.
- Kook S. Y., Lee K. M., Kim Y., Cha M. Y., Kang S., Baik S. H., Lee H., Park R and Mook-Jung I.High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5 XFAD mice.Cell Death and Disease. 2014;5.
- Rai A. R., Madhyastha S., Rao G. M., Rai R and Sahu S. S. A Comparison of Resveratrol and Vitamin C Therapy on Expression of BDNF in Stressed Rat Brain Homo genate. IOSR Journal Of Pharmacy. 2013;3(10):22-27.
CrossRef - Pang Y., Cai Z and Rhodes P. G. Disturbance of oligodendrocyte development, hypomyeli nation and white matter injury in the neonatal rat brain after intra cerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214.
CrossRef







