Manuscript accepted on :16 May 2018
Published online on: 08-06-2018
Husnul Khotimah1, Darwitri2, Tri Yuliyani2, Een Nuraenah2, Evi Zahara2, Umi Kalsum1, Nurdiana1 and Mohammad Muljohadi Ali1
1Laboratory of Pharmacology, Medical Faculty, Brawijaya University, Indonesia.
2Master of Midwifery, Medical Faculty, Brawijaya University, Indonesia.
Corresponding Author E-mail: husnul_farmako.fk@ub.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1438
Abstract
Centella asiatica (CA) is herbal medicine that used as traditional medicine including ayurvedic theraphy since hundreds years ago. This herb containa of pentacyclic triterpenoids such as asiaticoside, madecassoside, Asiatic acid and brahmoside that proved had anti-oxidant and anti-inflammatory properties. This research aims to know the effect of ethanolic extract of CA extract against the length of rotenone-induced zebrafish larvae through the free radicals.mechanism. This research used zebrafish larvae until 6 dpf that consists of 5 groups (controls, rotenon 12.5 ppb on 2 hpf-3 dpf, and group treatment given rotenone 12.5 ppb 2 hpf-3 dpf and 5 µg/mL extract with long exposure to start 2 hpf to 4, 5 and 6 dpf respectively). The body length measured on 3-6 dpf using software Image Raster v 3.0 from optilab v 2.0. Malondialdehyde (MDA), superoxide Dismutase (SOD), catalase were measured by ELISA on 6 dpf. The results showed rotenon can inhibit the growth of length > 2 standard deviation (SD) and CA extract may increased ithe body in 6 dpf which correction value was 99.6%. CA extract significantly decreased the levels of MDA, and increased the level of SOD and catalase (p=0.000). Ethanol extract of Centella asiatica may increase in length through the modulation of oxidative stress.
Keywords
Centella Asiatica; Catalase; Malondialdehyde; Rotenone; Superoxide Dismutase; Zebrafish
Download this article as:| Copy the following to cite this article: Khotimah H, Darwitri D, Yuliyani T, Nuraenah E, Zahara E, Kalsum U, Nurdiana N, Ali M. M. Centella Asiatica Increased the Body Length Through the Modulation of Antioxidant in Rotenone-Induced Zebrafish Larvae. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Khotimah H, Darwitri D, Yuliyani T, Nuraenah E, Zahara E, Kalsum U, Nurdiana N, Ali M. M. Centella Asiatica Increased the Body Length Through the Modulation of Antioxidant in Rotenone-Induced Zebrafish Larvae. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20653 |
Introduction
Rotenone is a natural pesticide extracted from the roots of tropical and sub-tropical plants derived from the leguminosae family and from the genus lonchocarpus in America, and Derris in Asia that can be used for insecticides, pesticides and piscisida.1 Rotenone as endocrine disrupting chemicals (EDCs) can disrupt hormonal homeostasis2 and decrease the amount of Adenosine Triphospate (ATP) and increase the production of reactive oxygen species (ROS).3 ROS interaction with polyunsaturated fatty acid (PUFA) causes lipid peroxidation with one of its products malondialdehyde (MDA).4 Antioxidants are reductant compounds that function to lower oxidation reactions and bind to free radicals that can inhibit cell damage.5 Antioxidants are classified into endogenous antioxidants and exogenous antioxidants. Endogenous antioxidants include superoxide dismutase (SOD) and catalase (CAT). SOD is the main antioxidant enzyme that plays a role in the elimination of oxidative stress.6 Catalase is an enzyme that plays a role in catalyzing the dismutation of hydrogen peroxide (H2O2) into water and oxygen.7
Centella asiatica has the main phytonutrients of triterpenoids that act as antioxidants in balancing the oxidants in cells so that oxidative stress can be prevented.5 In addition Centella asiatica contains nutrients such as macronutrients (proteins and carbohydrates) and micronutrients such as vitamins and minerals.8,9 Centella asiatica significantly reduced levels of malondialdehyde (MDA) and increased antioxidant enzyme levels i,e superoxide, catalase and glutathione peroxidase in diabetic rats.10
Previous studies have suggested 12.5 ppb rotenone can induced stunting with 98% confidence degree and administration of 5 μg/mL pre hatching (2-72 hpf) significantly increases insulin growth factor-1 (IGF-1) expression and increases body length.11 The purpose of this research is to know effect of ethanolic extract of Centella asiatica until the sixth day to rotenone-induced zebrafish larvae through the antioxidant mechanism.
Materials and Methods
Animal Treatment
The mature wild type zebrafish males and females were identified in the laboratory of Hydrology Faculty of fisheries and Marine Sciences University of Brawijaya Malang, Indonesia. Zebrafish are kept in semistatic 60 L tank.12 The water temperature was kept between 26-28°C, pH 6.8-7.5, and a lighting cycle 14:10 (dark: light).13 Fish were fed three times a day (Tetra Bit Color Tropical Flakes, Tetra Sales, Blacksburg, Germany).12
The zebrafish embryo was obtained from the fertilization of male and female parent with a ratio of 2:1. Zebrafish embryos 0-2 hpf (hour post fertilization) with criteria round, transparent, fertile, and not moldy. The number of larvae used was 30 larvae/group. All procedures have been approved by the Ethics Committee, Faculty of Medicine, Universitas Brawijaya (No. 403/EC/KEPK/12/2017). Zebrafish embryos were divided into 5 groups : controls (C), Rotenone (R), Rotenone + CA extract for 4,5 and 6 days respectively.
Embryonic Medium
Embrionic medium was made with concentration of 10x by CaCl 0.25 gr, KCl 0.15 gr, NaCl 5 gr, MgSO4 0.815 gr and 500 ml of aquadest (Modified from Cold Spring Harbor Laboratory Press).14
Extraction of Centella asiatica
Centella asiatica was certified from UPT Materia Medica, Batu, Malang East Java, Indonesia. The extraction was done using maceration method with 98% ethanol solvent.12
Rotenon and Extract Administration
Rotenone sigma (R8875) with purity of ≥95% dissolved in DMSO (dimethyl sulfoxide 1%) to obtain a stock solution of 2 x 107 μg/L (ppb). Rotenone was given at 12.5 ppb and Centella asiatica extract 5 mg/mL. The extracts were stocked with concentrations of 1 mg/mL. The medium was replaced daily.11
The Body Length Measurement
The body length measurements of zebrafish larvae were performed at 3-6 dpf. Larvae were observed using the Olympus SZ61 stereometry microscope that was connected to Optilab software version 2.0. The body length was measured from the tip of the nose (tip of the snout) to the base of the caudal fin using the calibrated Immage Raster software version 3.0.15
Measurements of Malondialdehyde,
The evaluation of MDA, SOD and Catalase levels was performed on day 6. The zebrafish larvae were euthanized by put in zebrafish larvae into ice water containing 5 parts of ice and 1 part of water for 40 minutes. The temperature of the ice water was held at a temperature of 0°C monitored using a thermometer.16
Zebrafish larvae (n=30 larvae/group) homogenized using 500 μL Ripa Buffer in glass homogenizer. Homogenate was centrifuged at 4°C, 2500 rpm for 20 min. Supernatant was taken for evaluation procedure. Malondialdehyde, SOD, and Catalase were examined in accordance with the ELISA Kit protocol (Bioassay Laboratory Technology, Shanghai, China), Cat.No E0156Ra (MDA), Cat.No E0168Ra (Cat) N. Cat E0869Ra (Catalase).
Statistical Analysis
Statistical analysis was performed by IBM ANOVA SPSS v23.0 and continued by LSD post hoc test with 5% confidence level. Normality testing was performed using the Shapiro-Wilk test and homogeneity testing was performed using Levene test.
Results and Discussion
Based on figure 1, at the 3 dpf the growth chart shows adjacent points in all groups. The result of statistical analysis of length comparison at age 3 dpf got p-value equal to 0247. So it can be concluded that there was no significant difference between all groups. Figure 2 showed that there was significant difference of the body length at 6 dpf among groups (p-value = 0.000). The rotenone group had the lowest body length compared to the control and treatment groups. This is in accordance with previous studies, where rotenon projections of 2.21 μg /L and 2.75 μg /L for 32 days significantly decreased the length of the body length in rainbow trout.17 Rotenone can decrease bone ossification in zebrafish larvae, which causes damage18. Increased ROS in bone cells causes bone growth disorders.19 Highly concentration of ROS in the body will induce RANKL (receptor activator of NF-κB ligand) to interact with RANK thus activating the RANKL pathway that may cause imbalance in the formation process and resorption of the bone.20 In addition, the increasing of ROS causes impairment in Insulin Growth Factor-I(IGF-I).21 IGF-I plays a role in mediating growth and development of somatic cells, including the muscles and bones during prenatal and post natal.22
 |
Figure 1: The average of body length at 3-6 dpf.
|
Rotenone group showed the average of body length had the shottest compared to others. Linier growth of R+CA 4 days, R+CA 5 days, dan R+CA 6 almost reach the control group.
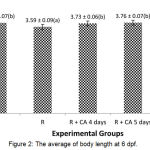 |
Figure 2: The average of body length at 6 dpf.
|
There were significant different among the groups (p =0.000). The group of R+CA 4 days, R+CA 5 days, and R+CA 6 days have the average of body length more than rotenone group and had no significant different to the control group (p>0.05).
Figure 3 showed that the rotenone group had higher MDA levels and lower SOD and catalase levels. Another study proved that rotenone 30 mg/kg for 60 days in mice significantly increased levels of MDA and reduced endogenous antioxidants such as GSH.23 Rotenone inhibit the mitochondrial complex I which causes a decrease in the amount of Adenosine Triphospate (ATP) so that the nucleus fails to divide and apoptosis occurs.24 Leakage of complex I results in the increasing of free electrons reacting to oxygen molecules resulting in superoxide (O2-) production.25 Superoxide will be converted to hydrogen peroxide (H2O2) and O2 by SOD as an endogenous antioxidant. Hydrogen peroxide will be convert by catalase to H2O and O2.26 Hydrogen peroxide is not reactive,27 but if H2O2 reacts with Fe2+ or Cu2+ (Haber-Weiss and Fenton reaction) to form a highly reactive hydroxyl (OH–) radical.26 Hydroxyl radicals can attack polyunsaturated fatty acid (PUFA) by lipid peroxidation process to form hydroperoxide.4 One of the secondary aldehydes produced by lipid peroxidation is Malondialdehyde (MDA)28 which is capable of inactivating many cellular proteins26.
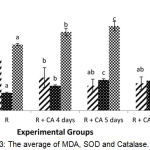 |
Figure 3: The average of MDA, SOD and Catalase.
|
There were significant different among the groups (p =0.000). CA extract significantly decreased the MDA level, and increased the antioxidant (SOD and Catalase) in zebrafish larvae-induced rotenon.
Research conducted on stunting children increased MDA levels and decreased the amount of antioxidants such as catalase, SOD and GSH.29 Stunting is a growth disorder in which the body length corresponds to <-2SD age based on the WHO child growth chart.30 Stunting children found normal body length at birth, no congenital abnormalities, and have the same body proportions with normal children.31
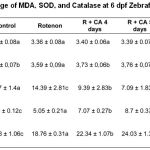 |
Table 1: The Average of MDA, SOD, and Catalase at 6 dpf Zebrafish larvae
|
Based on figure 3, 5 μg/mL Centella asiatica extract for 4, 5, and 6 dpf significantly decreased MDA level compared to rotenone group (p<0.05). Administation of Centella asiatica in this study was given from intrauterine to extra uterine. This refers to the occurrence of stunting starting from within the womb and continues until 2 years (first 1000 days of life).30 Figure 3 also showed that administration of ethanol in extract of Centella asiatica for 4,5 and 6 days significantly increased the SOD and Catalase levels (p<0.05). Administration of Centella asiatica extract for 4,5 and 6 days were able to correct the body length of 99.6%. Centella asiatica might increased the body length through the expression of osteoprotegerin (OPG) as receptor for osteoblast and decreasing the expression of RANKL (receptor activator of Nuclear kappa beta ligand) as indicator for osteoclastonegesis.32 Thus, the binding of OPG and RANKL inhibit of osteoclastogenesis process.20
Centella asiatica contains phytonutrients including of triterpenoids, carotenoids, flavonoids, alkaloids, glycosides, and essential oils.5 The high level of triterpen in Centella asiatica can provide antioxidant protection33. Asiaticoside contain in Centella asiatica by UHPLC (ultra high performance liquid chromatography) examination of 2.94 ppm.12 The ethanolic extract of Centella asiatica in tthis study came from the same simplia as the previous study (Khotimah, et al), so it is assumed to have the same asiaticoside content. In addition, flavonoids contained in Centella asiatica can act as an important antioxidant.5
Centella asiatica significantly decreases MDA and increases antioxidant enzymes, such as SOD, glutathione peroxidase, and catalase to protect the body from the ROS reactions.34 It has potential as a scavenger of superoxide free radicals, hydrogen peroxide, nitric oxide.35 This condition leads to a decrease in ROS production in the body, thus reducing MDA levels. MDA is an poly-unsaturated fatty acid oxidation product by free radicals hydroxyl and metabolite of cell components production.36 Another study proved that ethanolic extract of Centella asiatica stabilized free radicals by radical scavenger and show H2O2 scavenging activity.27 It also increase the activity of SOD, Catalase, and glutathione peroxidase, and glutathione reductase.37 Centella asiatica increases the expression of the NRF2 gene,38 which is the key transcription factor regulating the antioxidant response.39
Decreasing the MDA levels, elevated SOD and Catalase levels were followed by a significant increase in body length between treatment groups compared to the rotenone group (p-value <0.05). Administration of Centella asiatica showed non-significant body length to control (p-value> 0.05) with correction of body length at 6 dpf of 99.6%.
Conclusion
It can be concluded that Centella asiatica increased the body length in rotenone-induced zebrafish larvae. Administration of 5 μg/mL ethanol extract of Centella asiatica can decrease free radical activity with decrease MDA, and increase SOD and catalase level in rotenone-induced zebrafish larvae.
Acknowledgement
We would like to thanks to Mrs. Ferrida and Mr. Wahyudha Ngatiril Lady for the kindly help in laboratory.
References
- Turner L, Jacobson S and Shoemaker L. Risk assesment for piscicidal formulation of Rotenone. Compliance Services International. Lakewood. 2007; Pp.15-19.
- Diamanti-Kandarakis E, Bourguignon J.P, Guidice L.C, Hauser R, Prins G.S, Soto A.M, Zoeller R.T and Gore A.C. Endocrine-disrupting chemicals: an Endocrine society scientific statement. Endocrine Riview. 2009;30(4):293-342.
CrossRef - Radad K, Rausch W.D and Gille G. Rotenon induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochemistry International. 2006;49(4):379-386.
CrossRef - Burton G.J, and Jauniaux E. Oxidative stress. Best Practice & Research Clinical Obstetrics and Gynaecology. 2011;25(3):287–299.
CrossRef - Chandrika U.G, and Kumarab P.A.A.S.P. Gotu Kola (Centella asiatica): Nutritional properties and plausible health benefits. Advances in Food and Nutrition Research. 2015;76:1043-4526.
CrossRef - Fuji J, Luchi Y and Onaka Y. Review: Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reproductive Biology and Endocrinology. 2005;3:43.
CrossRef - Domínguez L, Sosa-Peinado A, and Hansberg W. Catalase envolved to concentrate H2O2 at its active site. Archives of Biochemistry and Biophysics. 2010;500:82-91.
CrossRef - Hashim P, Sidek H, Helan M.H.M, Sabery A, Palanisamy U.D and Ilham M. Triterpene composition and bioactivities of Centella asiatica. Molecules., 2011;16(2):1310-1322.
CrossRef - Joshi K and Chaturvedi P. Therapeutic efficiency of Centella asiatica (L.)Urb. An underutilized green leafy vegetable: An overview. International Journal of Pharma and Bio Science,2013; 4(1):135-149.
- Giribabu N, Srinivasarao N, Rekha S.S, Muniandy S and Salleh N. Centella asiatica attenuates diabetes induced hippocampal changes in experimental diabetic rats. Evidence-Based Complementary and Alternative Medicine. 2014;2014: 1-10.
- Cory’ah F.A, Nurdiana and Khotimah H. Pengaruh ekstrak etanol pegagan (Centella asiatica) pada model Stunting larva zebrafish (Danio rerio) dengan induksi rotenon melalui ekspresi Insuline Like Growth Factor-1 (IGF-1) dan Insulin Receptor Substrat (IRS). Universitas Brawijaya, Indonesia. 2017.
- Khotimah H, Sumitro S.B, Ali M and Widodo M.A. Standardized Centella asiatica increased brain- derived neurotrophic factor and decreased apoptosis of dopaminergic neuron in rotenone- induced zebrafish. GSTF Journal of Psychology (JPsych). 2015;2(1):22-27.
- Avdesh A, Chen M, Martin-iverson M.T. Mondal A. Ong D, Rainey-smith S and Martin R.N. Regular care and maintenance of a zebrafish (Danio rerio) laboratory : An Introduction. Journal of Visualized Experiments. 2012;69:1–8.
CrossRef - Protocol Zebrafish embryo medium. Cold Spring Harbor Laboratory Press. 2011. http://cshprotocols.cshlp.org/content/2011/8/pdb.rec12478.full?text_only=true. Accessed January 3, 2018.
- Primaditya V, Ali M and Khotimah H. Efek ekstrak etanol pegagan (Centella asiatica) pada Stunting larva zebrafish (Danio rerio) akibat induksi rotenon melalui peningkatan ekspresi GLUT 4 dan osteocalcin. Universitas Brawijaya, Indonesia. 2017.
- Strykowski J.L and Schech J.M. Effectiveness of recommended euthanasia methods in larval zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science. 2015;54(1):81-84.
- Bills T.D, Rach J.J and Marking L.L. Toxicity of rotenone to developing rainbow trout. S Fish and Wildlife Service. United States. 1988; Pp. 1-3.
- Primihastuti D, Ali M and Kalsum U. Pengaruh ekstrak etanol pegagan (Centella asiatica) pada osifikasi tulang dan osteoklastogenesis pada model stunting larva zebrafish (Danio rerio) yang diinduksi rotenon. Universitas Brawijaya, Indonesia. 2017.
- Riancho J.A, and Delgado-Calle J. Mecanismos de interaccion osteoblasto-osteoclasto. Reumaltologia Clinica. 2011;7:1-4.
CrossRef - Ha H, Kwak H.B, Lee S.W, Jin H.M, Kim H.M, Kim H.H and Lee Z.H. Reactive oxygen species mediate RANK signaling in osteoclast. Experimental Cell Research. 2014;301(2):119-127.
CrossRef - Backeljauw P, Bang P, Dunger D.B, Juul A, Le Bouch Y and Rosenfeld R. Insulin-like growth factor-I in growth and metabolism. Journal of Pediatric Endocrinology and Metabolism. 2010; 23(1-2):3-16.
CrossRef - Wood A.W, Duan C and Bern H.A. Insulin-like growth factor signaling ini fish. International Review of Cytology., 2005;243:215-285.
CrossRef - Zou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, Hu Z, Cai X, Yang J, Sun X, Lu W, Yan H, Chen J, Ye J, Yang J, Sun X, Lu W, Yan H, Chen J, Ye J, Shen J and Cao P. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Scientific Reports. 2016;6(32206):1-12.
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez J.A and Robinson J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. Journal of Biological Chemistry 2003;278(10):8516-8525.
CrossRef - Sanders L.H and Greenamyre J.T. Free Radical Biology and Medicine Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radical Biology and Medicine., 2013; 62: 111-120.
CrossRef - Birben E, Murat U, Sahiner M.D, Sackesen C, Erzurum S, and Kalayci O. Oxidative stress and antioxidant defense. WAO Journal. 2012;5:9-19.
- Raju D.C, Victoria T.D, Biji N, and Nikitha G. Evaluation of Antioxidant Potential of Ethanolic Extract of Centella asiatica Research J. Pharm. And Tech. 2015;8(9):1289-1293.
- Ayala A, Munoz M.F and Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanism of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:1-31.
- Aly G.S, Shaalan A.H, Mattar M.K, Ahmed H.H, Zaki M.E, and Abdallah H.R. Oxidative stress status in nutritionally stunted children. Egyptian Pediatric Association Gazette. 2014;62(1): 28-33.
CrossRef - de Onis M and Branca F. Childhood Stunting: A global perspective. Maternal and Child Nutrition. 2016;12:12–26.
CrossRef - Picasso B.C. A public health aproach to undernutrition in children under five and infants in Euthiopia: An Overview. The University of Arizona. 2016.
- Ariyati L.I.P, Ali M and Kalsum U. Pengaruh ekstrak etanol pegagan (Centella asiatica) terhadap terhadap ekspresi Osteoprotegri (OPG) dan Receptor Activator Nuclear Kappa-β Ligan (RANKL) pada stunting larva zebrafish (Danio rerio) yang diinduksi rotenon. Univertitas Brawijaya, Indonesia. 2017.
- Rahman M, Hossain S, Rahaman A, Fatima N, Nahar T and Uddin B. Antioxidant activity of Centella asiatica (Linn.) Urban : Impact of extraction solvent polarity. Journal of Pharmacognosy and Phytochemistry. 2013;1(6):27-32.
- Choi M.J, Zheng H.M, Kim J.M, Lee K.W, Park Y.H and Lee DH. Protective effects of Centella asiatica leaf extract on dimethylnitrosamine-induced liver injury in rats. Molecular Medicine Reports. 2016;14:4521-4528.
CrossRef - Sugunabai J, Jeyaraj M and Karpagam T. Analysis of functional compounds and antioxidant activity of Centella asiatica. World Journal Of Pharmacy and Pharmaceutical Sciences. 2015; 4(8):1982-1993.
- Devlin M.T. Bioenergetic and Oxidative Metabolism in Biochemistry with Clinical Correlations. 5 th ed. Wiley-liss. Canada. 2002; Pp. 590-592.
- Chen C.L, Tsai W.H, Chen C.J and Pan T.M. Centella asiatica extract protects again amyloid β1-40- induced neurotoxicity in neural cells by activating the antioxidave defence system. Journal of Traditional and Complementary Medicine. 2016;6:362-369.
CrossRef - Gray N.E, Harris C.J, Quinn J.F and Soumyanath A. Centella asiatica moldulates antoxidant and mitochondrial pathways and improves cognitives function in mice. J Ethnopharmacol. 2016;180:78-86.
CrossRef - Li W and Kong A.N. Moleculer of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48(2):91-104.
CrossRef







