Najam Siddiqi1, Tom Heming2, Asem Shalaby3, Mohammad Al-Kindi3, Fatima Al-Ghafri3 and Raheel Younas4
1Department of Anatomy and Neurobiology, Oman Medical College, PO Box 391, Sohar, Sultanate of Oman, 321.
2Department of Human Function, Oman Medical College.
3Department of Pathology, College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman.
4PNR Headquarters, Islamabad, Pakistan.
Corresponding Author E-mail: najam@omc.edu.om
DOI : https://dx.doi.org/10.13005/bpj/1214
Abstract
To measure the electromagnetic waves (RFW) emitted by different mobile phones and to further examine the effects of these waves on the living cells. TriField meter Model 100XE was used to measure the electromagnetic waves (mW/cm2) emitted by different mobile phones while receiving a call. To explore the effects of electromagnetic waves on living cells, developing chick embryo was selected for this study. 40 chick fertilized eggs were equally divided into a control and an exposed group. 20 fertilized eggs were placed in an egg incubator with a mobile phone (SAR US: 1.10W/kg (head) 0.47 W/kg body) in silent mode having vibration disable mode. Mobile was called for total of 50 minutes in 24 hours. 20 embryos each were sacrificed at day 10 and 15, and histology of liver was conducted. The control group, 20 eggs were incubated in the same conditions, having removed the battery from the phone. The TriField readings from different mobile phones were classified into four groups according to the strength of the waves. Downloading from the net using WiFi also revealed high intensity of the waves. Histology of the liver: Control group at day 10, showed developing hepatocytes, row not well formed, and few sinusoids in-between with few scattered RBSs. At day 15, well-formed anastomosing cords of hepatocytes with prominent nucleus were observed and well-formed sinusoids lined by epithelium showing well-formed RBCs can be seen clearly. In the exposed group at day 10, the sinusoids were less visible and necrosis of hepatocytes was seen. At day 15, in all the embryos, hepatocytes showed marked infiltration of fatty vacuoles in their cytoplasm, necrosis of few hepatocytes showing deeply stained nucleus, derangement of rows forming classic hepatic lobule, absence of sinusoids and few scattered RBSs. Different mobile phones showed emission of electromagnetic waves whose intensity was high in the old version of mobile phones. These waves affected the development of liver in the chick embryo; hepatocytes revealed fatty change and derangement of sinusoids in the exposed group. This study concludes that the electromagnetic waves of the mobile phones affected the proliferation of living cells in chick embryo.
Keywords
Chick Embryo; FattyLiver; Change Electromagnetic Waves; Mobile phone;
Download this article as:| Copy the following to cite this article: Siddiqi N, Heming T, Shalaby A, Al-Kindi M, Al-Ghafri F. Mobile Phone Electromagnetic Waves Causing Fatty Change in the Hepatocytes of the Developing Chick Embryo: Are Smart Phones too Close for Comfort?. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Siddiqi N, Heming T, Shalaby A, Al-Kindi M, Al-Ghafri F. Mobile Phone Electromagnetic Waves Causing Fatty Change in the Hepatocytes of the Developing Chick Embryo: Are Smart Phones too Close for Comfort?. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16719 |
Introduction
Smart phones have added a new dimension into our lives. We are busier with our mobile than talking to the person sitting next to us. Parents give their mobiles to their two-year old to play with instead of toys. Before traveling we make sure that the mobile charger is not left behind. Indeed smart phones have dramatically invaded into our lives; kids and teenagers spend more time playing with mobiles than playing outside. But do we know that we are risking our health from spending too much time with smart phones? They use non-ionizing low frequency electromagnetic waves for communication. These waves were initially thought to be harmless to the humans, however, now scientific research has revealed that these waves may cause damage to the living cells. Smart phone emits radio waves while in use which include downloading data from the internet.1,2 Fetus and children are more radiosensitive than adults due to the presence of embryonic stem cells.3,4,5,6,7 A child born in this era will start electromagnetic waves exposure as early as two years old and will remain in this environment until he dies. Divan et al. reported behavioral problems in children who were exposed to prenatal and postnatal cell phone9
Hypothesis of this research conducted at Oman Medical College, is that electromagnetic waves emitted by mobile phone affects the normal functioning of the living cells.
Objectives
To measure the strength of the electromagnetic waves emitted by different mobile sets
To study the effects of these electromagnetic waves on the living cells.
Material and methods
This study was approved by the Institutional Review (Research) Board of Oman Medical College.
Different models of mobile sets from different companies were randomly selected to measure the RFW emission during receiving a call. This included older version of mobile sets and smart phones. TriField Meter was placed next to the mobile phone while it was receiving a call. The TriField meter will show a deflection of the needle towards the right which was ranging from .01 to 1 mW/cm.2 Pictures were taken to record the position of the needle showing the intensity of the electromagnetic waves.
Animal experiment
‘Cobb’ (Gallus gallus domesticus) breed zero-day fertilized chicken eggs were acquired from Sohar poultry, by applying pre-fixed inclusion and exclusion criteria. Chick embryo model was previously extensively used as an animal model to access the effects of electromagnetic waves10-19
A 30-egg incubator (Egg incubator Model EH-35, Sino-PFE Company, China) with automatic temperature, humidity control and forced air ventilation was used. It was also equipped with special egg holders with automatic egg rotation capability which was fixed at ten rotations per day. The mobile phone was placed in the center of the incubator under the egg holder so that the farthest egg was within a radius of 16 cm . The temperature was set at 37 degrees and the humidity at 50-60%. 30 eggs were placed at one time in the egg holders.
The experiment was done twice and the specimens were sent to two different laboratories for histological preparations. It was partially blinded. For each experiment, 40 fertilized eggs were randomly divided into the two groups, control and experimental group. One incubator was used carrying 20 eggs at a time; one wave of exposed group experiment and one wave of control group.
Fresh fertilized chicken eggs were exposed to RFW emitted by a mobile phone during embryonic development and compared with the control eggs, which were not exposed to RFW. A popular mobile phone and service provider was selected with 1800 MHz frequency, power of 0.47 W/kg body and SAR 1.10 w/KG (head). A TriField Meter, model 100XE was used to detect the strength of RFW of the mobile phone during the experiment (Fig.1).
Experimental group
20 fertilized eggs were incubated in the incubator with the mobile phone in silent mode with the vibration mode disabled. The distance of all the eggs form the mobile phone was maintained within one wavelength (approximately 16.5 cm) of the emitting 1800 MHz frequency electromagnetic waves.14 The mobile phone was rung from another mobile phone for 5 min, ten times daily with an exposure-free period in between the calls. No calls were made at night. The total daily exposure duration was 50 minutes in each 24 hours starting from day 1. The eggs were sacrificed at day 10 (maximum exposure time 500 minutes) and day 15 (total exposure time 750 minutes). For each set of experiments, for both the control and exposed groups, 20 eggs were placed in the incubator, and 10 eggs were sacrificed at day 10 and 15.
 |
Figure 1.a: A 30-egg incubator b) TriField Meter showing high electromagnetic waves from mobile during call receiving mode
|
On the scheduled day of sacrifice, in each egg, a small hole was first made in the shell and then a portion of the shell was carefully cut by scissors and removed. The embryo was dissected from the membranes and its survivability noticed by either movements of the limbs or beating of the heart. Liver was dissected and placed in formalin
Control Group
20 eggs were incubated at same conditions in the same incubator. The mobile phone was turned off, battery removed and placed in the middle of the incubator. The embryos were examined just as in the experimental groups at days 10 and 15.
5 specimens from day 10 and day 15 each in the exposed group and control groups were selected for histological preparation. One set of 20 specimens were sent to Department of Pathology, Royal Hospital, Muscat and the other set of 20 specimens were sent to Department of Pathology, Sultan Qaboos University, Muscat. They were partially blinded.
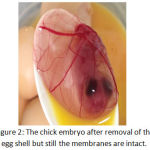 |
Figure 2: The chick embryo after removal of the egg shell but still the membranes are intact.
|
Results
The TriField meter revealed different intensity of electromagnetic waves from different mobile sets. The intensity of RFW was divided into four groups as follows:
 |
Figure 3: Tri Field meter showing the intensity of the electromagnetic waves by deflecting the needle towards right
|
Group 1: .01-.1 mW/cm2
Group 2: .1- .2 mW/cm2
Group 3: .2-1 mW/cm2
Group 4: more than 1 mW/cm2
All the old mobile sets were placed in Group 4 showing highest levels of radiation (≥ 1mW/cm2) which is recommended dangerous to health. Mostly the smarts phones were in groups 1 (.01-.1 mW/cm2), 2 (.1- .2 mW/cm2) and 3 (.2-1 mW/cm2), but few in group 4. It was further observed that downloading from the net using WiFi also results in high levels of radiations.
 |
Figure 4: Tri Field meter showing the needle moving towards the right which means increasing the intensity of the electromagnetic waves
|
Results from Royal Hospital, Muscat
Control group
At day 10, control group showed hepatocytes with central large nucleus and showing prominent nucleolus. They were arranged in rows, showing central vein with few RBCs, and the beginning of sinusoid formation. The classical hepatic lobule was not yet fully formed. At day 15, control group revealed well-formed anastomosing cords of hepatocytes with prominent nucleus around the sinusoids lined by epithelium cells and scattered RBCs.
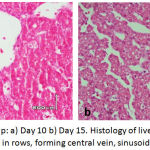 |
Figure 5: Control Group: a) Day 10 b) Day 15. Histology of liver showing normal hepatocytes arranged in rows, forming central vein, sinusoids and scattered RBCs
|
Exposed group
At day 10, in all the specimens, the hepatocytes were observed with prominent nucleus, central vein, and sinusoids. However, RBCs were scattered all over the hepatocytes. At day 15, the structure of hepatic classic lobule was completely destroyed. Hepatocytes were not lining in row, many hepatocytes showed signs of necrosis. Majority of hepatocytes were showing fat vacuoles in the cytoplasm which pushed the nucleus to one side. Sinusoids were completely disorganized and no lining epithelium was seen. RBCs were scattered all over the specimen.
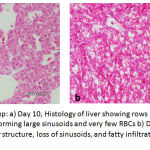 |
Figure 6: Exposed Group: a) Day 10, Histology of liver showing rows of hepatocytes which was not clearly seen, forming large sinusoids and very few RBCs b) Day 15, showing marked destruction of the liver structure, loss of sinusoids, and fatty infiltration in the hepatocytes with necrotic cells
|
Results from SQU, Muscat
Control group
At day 10, hepatocytes were seen with rounded central nucleus and nucleoli. They were lying in rows with spaces in-between to form the sinusoids. Central veins with few RBCs and portal area was observed. At day 15, typical structure of the liver was apparent. Well-formed hepatic lobules formed by rows of hepatocytes and sinusoids lined with epithelium in between with increase number of RBCs were clearly seen.
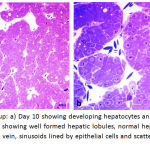 |
Figure 7: Control Group: a) Day 10 showing developing hepatocytes an sinusoids in between with RBCs b) Day 15, showing well formed hepatic lobules, normal hepatocytes arranged in rows, forming central vein, sinusoids lined by epithelial cells and scattered well-formed RBCs inside the sinusoids.
|
In the exposed group at day 10 and day 15, many hepatocytes the nucleus was absent or pushed to the side and without prominent nucleolus. The hepatocytes were seen in rows with sinusoids in-between, however, marked infiltration of the fat vacuoles was observed in the cytoplasm of hepatocytes. The sinusoids were formed showing lining epithelial cells and RBCs This signifies the beginning of fatty change.
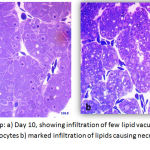 |
Figure 8: Exposed Group: a) Day 10, showing infiltration of few lipid vacuoles in the hepatocytes and few necrotic hepatocytes b) marked infiltration of lipids causing necrosis of the hepatocytes
|
Discussion
We are now living in a world totally depended on smart phones. Most of us don’t realize that these mobile phones are emitting electromagnetic waves all the time and we are living in an environment in which we are surrounded by these waves. Are these waves which are invisible to naked eye totally safe to our body is a big question. Are the babies as old as two years watching cartoons on such devices for hours every day safe from this environment hazard? Are the teenagers spending most of their evening time on computer games instead of outdoor activities or sports are growing normally? The pregnant women unknowingly sleeping with the mobile next to her abdomen will not be affecting the growing embryo? And of course the adults who are chatting all the time with their loved ones and sending pictures using what’s app are not prone to more diseases in the old age?
Measuring mobile phones RFW strength
To answer these questions and to confirm the emission of RFW from different mobile sets and to understand its strength, we decided to measure the RFW from different mobile sets using a TriField meter. It was found that the old mobile sets were emitting very high intensity of RFW which the triField meter showing above 1mW/cm2. Absolute hazard thresholds have not been established yet, however, studies suggest that RFW above 0.1mW/cm2 may not be safe. Our study revealed that most of the smart mobile sets are emitting RFW above 0.1mW/cm2. According to our classification, only group 1 comes under 0.1mW/cm2; groups2, 3 and 4 are all above this threshold.
Radio waves effect living cells
In this experimental study, it was found that electromagnetic waves caused fatty change in the hepatocytes of the developing chick embryos. In fatty liver, there is increase in lipid droplets in cytoplasm of the hepatocytes suggesting that cells are under oxidative stress when exposed to electromagnetic waves.20,21,22 The damage is dose dependent.14 Lahijani et al had similar results showing abnormal lipid droplets in the hepatocyte cytoplasm and pushing the nuclei to one side.23 Similar results were reported in rats and rabbits.23,24 The breakdown of fat in the liver may be disrupted by radiation exposure, which may be similar to alcoholism, malnutrition, poising and pregnancy. Fatty change is the beginning of injury to the hepatocytes, showing increase vacuoles filled with triglyceride fat, a sign of abnormal metabolism which may be due to production of oxygen radicles species in the hepatocytes.20,21,22 Many authors have reported different effects of electromagnetic waves on the chick embryo which increases mortality of the developing chick embryo and resulted in malformations.10-19
Mobile phone radiation induces reactive oxygen species and DNA damage in human sperm, affecting genes, cell membrane function and signal transduction.26-29 Different theories have been postulated regarding the effects of radio waves on the biology of living cells. Rao et al recently provided new evidence supporting the theory that radio waves affect the plasma membrane.30 Radio waves also induce oxidative stress, NADH oxidase enzyme stimulation, which might play a key role in the various cellular adverse effects observed in in vitro studies.31-38 As a consequence of increased levels of free radicals, various cellular and physiological processes can be affected including gene expression, release of calcium from intracellular storage sites, cell growth, and apoptosis. Radio wave effects on genes have also been reported resulting in signal transduction effects and alterations in membrane structure and function, metabolic effects associated with free-radical production.36-38
Recently, increase incidence of gliomas in Sweden, thyroid cancer in Korea, and other malignant brain tumors were reported, and it was associated with long term use of mobile phones.39-43
It is important that to realize that smart phones are not 100% safe hence caution is necessary to avoid much use.
Conclusion
Electromagnetic waves exposure to developing chick embryo has caused fatty change in the liver at 15th day of development. This result is in agreement with other researchers showing the same effect in liver of chick embryo and other animals. Clinical studies reported by other authors also associate brain tumors with excessive use of mobile phones. Hence it is quite clear that these electromagnetic waves are producing damage to the living cells. Further studies should be carried out to fully understand the mechanism of this fatty change in the hepatocytes when exposed to RFW.
Take home message
Keep mobile phones at least 2 feet away from your body.
Use headphones or speakers while talking.
Avoid long conversation on mobiles; use land line phones.
Pregnant women and children should use mobile phones only in emergency.
Mobile companies should inform the public of the RFW hazards.
More research should be done to make the mobile phones safer.
Acknowledgment
We are thankful to Dr. Susan Thomas, Department of Pathology, Royal Hospital, Mr Trevor Wicks, in-charge of histology preparations at pathology laboratory and Dr. Syed Mohammad Saud for collaborating in this project. We are also grateful to Dr. Irfan Ullah, Department of Pediatrics, Sultan Qaboos University, Oman for his continuous support. There is no conflict of interest and it was funded by OMC.
References
- Samkange-Zeeb F., Blettner M. Emerging aspects of mobile phone use. Emerging Health Threats Journal. 2009;2:2-8.
CrossRef - Blake L. B., Lai H. Biological effects from exposure to electromagnetic radiation emitted by cell tower base stations and other antenna arrays. Environ Rev. 2010;18:369-397.
CrossRef - Leitgeb N. Mobile phones: are children at higher risk? Wien Med Wochenschr. 2008;158(1-2):36-41.
CrossRef - Kheifets L., repacholi M., saunders R., van deventer E. The sensitivity of children to electromagnetic fields. Pediatrics. 2005;116:303-13.
CrossRef - Maisch D. Children and mobile phones. Is there a health risks? Journal of Australasian College of Nutritional and Environmental Medicine. 2003;22:3-8.
- Siddiqi N., Heming T. Effects of mobile phone radiofrequency waves (RFW) on embryonic stem cells. The FASEB Journal. 2013;27(1):753.
- Schüz J. Mobile phone use and exposures in children. Bioelectromagnetics. 2005;7:S45-50.
CrossRef - Abramson M. J., Benke G. P., Dimitriadis C et al. Mobile telephone use in associated with changes in cognitive function in young adolescents. Bioelectromagnetics. 2009;30:678-86.
CrossRef - Divan H. A., Kheifets L., Obel C., Olsen J. Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology. 2008;19(4):523-9.
CrossRef - Ingole I., Ghosh S. K. Exposure to radio frequency radiation emitted by cell phone and mortality in chick embryo (Gallus domesticus). Biomedical Research. 2006;17:205-210.
- Juutilainen J., Harri M., Saali K et al. Effects of 100 Hz magnetic fields with various waveforms on the development of chick embryo. Radiat Environ Biophys. 25:65-74.
CrossRef - Ubeda A., leal J., trillo M. A et al. Pulse shape of magnetic fields influences chick embryogenesis. J Anat. 137:513-536.
- Siddiqi N., John C. M., Saud M. S et al. Effects of Mobile phone 1800 Hz electromagnetic field on the development of chick embryo—A pilot study. International Conference on Chemical, Environmental and Biological Sciences CEBS Dubai (UAE). 2015;18-19.
- Zareen N., Khan M. Y., Minhas L. A. Dose related shifts in the developmental progress of chick embryo exposed to mobile phone induced electromagnetic fields. J Ayub Med Coll. 2009;21(1):130–4.
- Ubeda A., Leal J., Trillo M. A., Jimenez M. A., Delgado J. M. Pulse shape of magnetic fields influences chick embryogenesis. J Anat. 1983;137:513-536.
- Ubeda A., Trillo M. A., Chacon L., et al. Leal J. Chick embryo development can be irreversibly altered by early exposure to weak extremely-low-frequency magnetic fields. Bioelectromagnetics. 1994;15:385-398.
CrossRef - Youbicier-Simo B. J., Lebecq J. C., Giaimis J., et al. Mortality of chicken embryos continuously exposed under GSM cell phone and validation of the effectiveness of a protective device. International conference on cell tower siting, Salzburg, Austria. 2000;233-235.
- Al-Qudsi F., Azzouz S. Effects of electromagnetic radiation on chick embryo development. Life Science Journal. 2012;9:983-991.
- Farrell J. M., Litovitz T. L., Penafiel M., Montrose C. J., Doinov P., Barber M., Brown K. M., Litovitz T. A. The effect of pulsed and sinusoidal magnetic fields on the morphology of developing chick embryos. Bioelectromagnetics. 1997;18:431-438.
CrossRef - Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 346:1221-1231.
CrossRef - Reddy J. lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Amer J Physiol Gastrointest Liver Pgysiol. 290:852-858.
- Ibrahim M., El-Ashry., Ali E. The influence of 50 Hz magnetic field on liver function. Rom J Biophys. 18:113-122.
- Lahijani M. S., Tehrani D. M., Sabouri E. Histopathology and ultrastructural studies on the effects of electromagnetic fields on the liver of preincubated white Leghorn chicken embryo. Electromagnetic Biology and Medicine. 2009;28:391-413.
CrossRef - Atti A. A., Yehia M. A. Histological, ultrastructural and immunohistochemical studies of the low frequency electromagnetic field effect on thymus, spleen and liver of albino swiss mice. Pak J Bio Sci. 2002;5(9):931-937.
CrossRef - Dini P. Post-continuous whole body exposure of rabbits to 60 MHz electromagnetic fields: Effects on liver, spleen and brain. Radiat Environ Biophys. 44:51-59.
- Aitken R. J., Bennetts L. E., Sawyer D., Wiklendt A. M., King B. V. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germ line. Int J Androl. 2005;28:171–179.
CrossRef
- De Iuliis N. G., Newey J. R., King V. B., et. al., Mobile phone radiation induces Reactive Oxygen Species production and DNA damage in human spermatozoa in Vitro. PLoS One. 2009;4:e6446.
CrossRef - Xu S., Zhou Z., Zhang L., Yu Z., Zhang W., Wang Y., Wang X., Li M.,Chen Y., Chen C.,He M., Zhang G., Zhong M. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Research. 2010;1311:189.
CrossRef - Spadaro J. A., Bergstrom W. H. In vivo and in vitro effects of a pulsed electromagnetic field on net calcium flux in rat calvarial bone. Calcif Tissue Int. 2002;70:496–502.
CrossRef - Rao V. S., Titushkin I. A., Moros E. G., et. al. Non-thermal effects of radiofrequency-field exposure on calcium dynamics in stem cell-derived neuronal cells: elucidation of calcium pathways. Radiat Res. 2008;169(3):319-329.
CrossRef - Dasdag S., Akdag Z. M. The link between radiofrequencies emitted from wireless technologies and oxidative stress. Journal of Chemical Neuroanatomy. 2016;75:85.
CrossRef - Megha K., Deshmukh S. P., Banerjee D. B.,Tripathi K. A., Ahmed R.,Abegaonkar P. M. Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. Neuro. Toxicology. 2015;51:158.
CrossRef - Mailankot M., Kunnath A. P., Jayalekshmi H., Koduru B., Valsalan R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics (Sao Paulo). 2009;64(6):561-5.
CrossRef - Ragy M. M. Effect of exposure and withdrawal of 900-MHz-electromagnetic waves on brain, kidney and liver oxidative stress and some biochemical parameters in male rats.Electromagnetic Biology and Medicine. 2015;34(4):279.
- Mohamadin M. A., Elberry A. A., Mariee D. A., Morsy M. G., Al-Abbasi A. F. Lycopene attenuates oxidative stress and heart lysosomal damage in isoproterenol induced cardiotoxicity in rats: A biochemical study. Pathophysiology. 2012;19(2):121.
- Verschaeve L., Heikkinen P., Verheyen G., Van Gorp U., Boonen F., Plaetse F. V., Maes A., Kumlin T., Mäki-Paakkanen J., Puranen L., Juutilainen J. Investigation of co-genotoxic effects of radiofrequency electromagnetic fields in vivo. Radiat Res. 2006;165:598–607.
CrossRef - Donato A., Ceci P., Cannavo A., Tomei F., Naro F. Low power microwave interaction with phospholipase C and D signal transduction pathways in myogenic cells. Cell Biol Int. 2004;28:683–688.
CrossRef - Lantow M., Schuderer J., Hartwig C., Simkó M. Free radical release and HSP70 expression in two human immune-relevant cell lines after exposure to 1800 MHz radiofrequency radiation. Radiat Res. 2006;165:88–94.
CrossRef - Hardell L., Carlberg M. Mobile phone and cordless phone use and the risk for glioma – Analysis of pooled case-control studies in Sweden, 1997-2003 and 2007-2009. Pathophysiology. 2015;22(1):1-13.
CrossRef - Chang-Mo O., Kyu-Won J., Young-Joo W., Shin A., Hyun-Joo K., Jin-Soo L. Age-Period-Cohort Analysis of Thyroid Cancer Incidence in Korea. Cancer Research and Treatment. Official Journal of Korean Cancer Association. 2015;47(3):362-369.
- Hardell L., Carlberg M., Söderqvist F., Mild K. H. Meta-analysis of long-term mobile phone use and the association with brain tumours. Int J Oncol. 2008;32(5):1097-103.
CrossRef - Hardell L., Carlberg M., Söderqvist F., Mild K. H. Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol. 2013;43(6):1833-45.
CrossRef - Elliott P. Mobile phone base stations and early childhood cancers: case-control study. BMJ. 2010;340:3077-3090.
CrossRef








