Manuscript accepted on :March 03, 2017
Published online on: --
Plagiarism Check: Yes
Shubhangam Sharma1, Hemlata Verma2 and Manish Jain3
1Department of Pharmacology, M LB Medical College, Jhansi, UP, India.
2Department of Pharmacology, Gandhi Medical College, Bhopal, MP, India.
3Community medicine people dental academy Bhopal MP India.
Corresponding Author E-mail: vkverma0505@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1094
Abstract
Statins are the most widely prescribed drugs for the correction of dyslipidaemia and prevention of cardiovascular events in patients of Metabolic syndrome, so early detection of Adverse drug reactions(ADR) becomes necessary. This prospective, comparative study done in OPD setting in a tertiary care centre of central India aimed at finding out the ADRs of low dose atorvastatin in patients with metabolic syndrome versus the usual care group. Patients satisfying NCEP-ATPIII criteria for metabolic syndrome were divided into two groups in which one received statin therapy (Group A) and other did not(Group B). A total of 33 ADRs were recorded out of which 26 (38.23%) were in Group A and 7 (31.81%) were in Group B, with no significant difference in the ADRs recorded in both the groups. In patients with statin therapy, most common adverse effects were dyspepsia (10.29%) and elevation of hepatic transaminases(10.29%), followed by constipation (5.8%), sleep disturbances, myalgia and headache (2.9%). The adverse effect profile of group B was nearly similar with dyspepsia in 3 cases (13%) followed by constipation in 2 cases (9%) ,elevation in hepatic transaminases and headache in 1 case (4.5%). Definite (certain) relationship was established in 12% patients while probable in 23% and 65% ADRs were categorized as possible. Severity assessment recorded 73% of the total ADR as mild, 23% as moderate and only 3.8% as severe. Although statins are one of the safest medications for dyslipidaemia and metabolic syndrome as also proved in the present study; further studies of ADRs is needed for early detection, prevention and management of ADRs and reduced morbidity.
Keywords
ADRs; Atorvastatin; Metabolic syndrome; Dyslipidemia
Download this article as:| Copy the following to cite this article: Sharma S, Verma H, Jain M. Study of Adverse Drug Reaction of Low dose Atorvastatin in Patients With Metabolic Syndrome and Comparison With the usual Care Group. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Sharma S, Verma H, Jain M. Study of Adverse Drug Reaction of Low dose Atorvastatin in Patients With Metabolic Syndrome and Comparison With the usual Care Group. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13710 |
Introduction
Metabolic syndrome (MetS) is a constellation of cardiometabolic risk determinants comprising of obesity (Central adiposity), glucose intolerance and insulin resistance, dyslipidaemia (including hypertriglyceridemia, increased free fatty acids and decreased HDL–Cholesterol) & hypertension.(1,2) Statins are one of the major pharmacotherapeutic modalities for lipid management which also reduce coronary events in population at risk and in patients with stable coronary disease. [3,4] These are the most effective and best tolerated agents for the treatment of dyslipidemia. These drugs are competitive and reversible inhibitors of HMG-CoA reductase which plays a central role in the production of cholesterol in the liver. Because statins are similar to HMG-CoA on a molecular level they take the place of HMG-CoA in the enzyme and reduce the rate by which it is able to produce mevalonate, the next molecule in the cascade that eventually produces cholesterol,as well as a number of other compounds.Their predominant action is to reduce circulating levels of low-density lipoprotein (LDL) cholesterol, they also increase high-density lipoprotein (HDL) cholesterol and reduce triglyceride and VLDL concentrations.(4)
Mevalonate not only acts as precursor of cholesterol but also serves as a precursor for non-steroid isoprenoids such as CoQ10, heme-A, and farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). These intermediates of mevalonate pathway impact the benefits as well as risk of statins.(5) An array of additional risk factors for statin AEs are those that amplify (or reflect) mitochondrial or metabolic vulnerability, such as metabolic syndrome factors, thyroid disease, and genetic mutations linked to mitochondrial dysfunction. (6) Characteristics predisposing individuals to statin adverse effects include but are not limited to multiple or serious comorbidities, including impaired renal or hepatic function, history of previous statin intolerance or muscle disorders, unexplained ALT elevations ≥3 times ULN, patient characteristics or concomitant use of drugs affecting statin metabolism and also age >75 years.(7)
Adverse drug reaction and quality of life are most important in terms of patient health. For statins, as for all medications, vigilance for potential AEs is imperative. Recognition of potential statin AEs is needed and may be fostered by an improved awareness both of relevant literature and of its limitations. Most statin adverse effects, including the musculoskeletal (rhabdomyolysis) and gastrointestinal systems are dose-related(8). That is, higher doses bring increased risks. Also dose-related are elevations in plasma fibrinogen levels(9) and liver enzyme indices (10) .Each doubling of the statin dosage also doubles the incidence of liver enzyme elevations that exceed three times the upper limit of normal. Hence, rational dosing is key with statins for not only achieving target low-density cholesterol (LDL-C) levels, but also for avoiding adverse effects.
Physician awareness of statin AEs is reportedly low even for the AEs most widely reported by patients. Awareness and vigilance for AEs should be maintained to enable informed treatment decisions, treatment modification if appropriate, improved quality of patient care, and reduced patient morbidity. Hence this study was undertaken to study the adverse effect profile of low dose atorvastatin in patients of metabolic syndrome, a more vulnerable group for statin AEs.
Methods and Materials
This prospective and randomized observational study was conducted in the OPD patients at a tertiary care hospital in Bhopal. This study has been conducted observing ethical guidelines of Biomedical research on human participants following ethical review procedures, general ethical issues enshrined therein for prospective studies and after approval from the institutional ethical committee, as required.
Table 1: Age Group Wise Distribution Of Cases
| S. No. | Age group (yrs) | Group A (n=68) | Group B (n=22) | Total | |||
| No. | % | No. | % | No. | % | ||
| 1 | 21-30 | 6 | 8.8 | 1 | 4.5 | 7 | 7.7 |
| 2 | 31-40 | 8 | 11.76 | 4 | 18.18 | 12 | 13.33 |
| 3 | 41.50 | 27 | 39.7 | 9 | 40.9 | 36 | 40 |
| 4 | 51-60 | 20 | 29.41 | 6 | 27.27 | 26 | 28.8 |
| 5 | 61-70 | 7 | 10.29 | 2 | 9 | 9 | 10 |
| Total | 68 | 100 | 22 | 100 | 90 | 100 | |
Inclusion Criteria
Patients ready to give consent for the study.
Age group 20-70 years.
Patients satisfying at least 3 parameters of metabolic syndrome as per NCEP ATP-III (National Cholesterol
Education Program Adult Treatment Panel III ) criteria.
Exclusion Criteria
Patients not ready to give consent for study.
Age less than 20 years and greater than 70 years.
Patients with recent Myocardial infarction.
Pregnancy.
Patients already taking statins or other hypolipidaemic drugs.
Grouping of Patients
Patient Were Divided into 2 Groups –
Group A-Patients satisfying at least 3 parameters of metabolic syndrome as per the NCEP ATP-III criteria, who received statin therapy (Atorvastatin 20 mg/day).
Group B-Patients satisfying at least 3 parameters of metabolic syndrome as per the NCEP-ATP-III criteria, but did not take statin therapy due to any of the following reasons.
Non-compliance.
Contra-indications.
A total of 90 cases were studied in which group A (patients receiving statin therapy) included 68 cases (n=68) and group B (patients not taking statin therapy) included 22 cases (n=22). Both the groups also received target driven treatment for hypertension and elevated glucose, as required The cases were followed up for 3 months.
Evaluation and Statistical Analysis
The ADRs were recorded and summarized in a tabulated form and represented in percentages; Chi square value was calculated and also, a p-value of less than 0.001 was considered statistically significant. Causality assessment was done by using WHO (UMC) causality assessment scale. Severity assessment was done on a scale of mild, moderate and severe.
 |
Figure 1: Causality Assessment (WHO-UMC) of ADRs |
Results and Discussion
During the study a total of 90 patients having metabolic syndrome as per the NCEP-ATP-III criteria were enrolled, of which 68 were in Group A –who received statin therapy and 22 in Group B- who did not receive statin therapy. The characteristics of the patients in both the groups in terms of age group, sex wise distribution, lipid profile, liver function were comparable at baseline. Majority of patients were in the age group 41-60 years in both the groups. There were more females as compared to males in both the groups. There were 55.88% females in group A and 54.5% females in group B. Overall, there were 55.5% females in both the groups collectively. A high prevalence of metabolic syndrome (41.1%) in urban Asian Indian adults (Age 20-75 years) in Chennai was reported by Ramchandran A et al (2003); which was more common in women than in men (45.5% Vs 36.1%)(11) Similar observations have been recorded in this study.
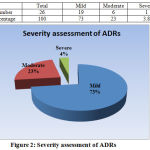 |
Figure 2: Severity assessment of ADRs
|
Table 2: Sex Wise Distribution of Cases
| S. No. | Sex | Group A (n=68) | Group B (n=22) | Total | |||
| No. | % | No. | % | No. | % | ||
| 1 | Males | 30 | 44.11 | 10 | 45.5 | 40 | 44.5 |
| 2 | Females | 38 | 55.88 | 12 | 54.5 | 50 | 55.5 |
| Total | 68 | 100 | 22 | 100 | 90 | 100 | |
The patients were assessed of having metabolic syndrome according to the NCEP-ATP-III criteria in accordance to which it was found that a total of 74.4% patients were having centripetal obesity, 82.3% of total patients were having high B.P. (B.P. >130/85 mmHg) and 75.5% of the patients were having high fasting plasma glucose levels (FPG >110 mg/dl) in both the groups collectively.
Table 3: Changes in Level of Hepatic Transaminases in Patients of Group A and B
| S.No. | Hepatic Transaminases | Group A (n=68) | Group B (n=22) | ||
| No. | % | No. | % | ||
| 1 | No change | 49 | 72 | 20 | 90.9 |
| 2 | Mild Change | 12 | 17.6 | 1 | 4.5 |
| 3 | Moderate increase | 6 | 8.8 | 1 | 4.5 |
| 4 | Significant increase | 1 | 1.4 | 0 | 0 |
| Total | 68 | 100 | 22 | 100 | |
Chi square value: 3.26; Significance P Value: 0.32(Not Significant)
The adverse effect analysis in our study revealed that the overall incidence of adverse effects was similar among both the groups and no statistically significant difference was observed (p value>0.001). Most common adverse effects were gastrointestinal system related. A total of 26 (38.23%) ADR were recorded in Group A and a total of 7 (31.81%) ADR were recorded in group B.
Table 4: Adverse Effects Observed in Patients of Group A and B.
| S.No. | Adverse Effect | Group A (n=68) | Group B (n=22) | ||
| No. | % | No. | % | ||
| 1 | Dyspepsia | 7 | 10.29 | 3 | 13 |
| 2 | Constipation | 4 | 5.8 | 2 | 9 |
| 3 | Pain in abdomen | 1 | 1.4 | 0 | 0 |
| 4 | Sleep Disturbance | 2 | 2.9 | 0 | 0 |
| 5 | Myalgia | 2 | 2.9 | 0 | 0 |
| 6 | Headache | 2 | 2.9 | 1 | 4.5 |
| 7 | Mood & Behaviour changes | 1 | 1.4 | 0 | 0 |
| 8 | Elevations in Hepatic transaminases | 7 | 10.29 | 1 | 4.5 |
| Total | 26 | 38.23 | 7 | 31.81 | |
Chi square value: 3.23; Significance P Value: 0.863(Not Significant)
In patients with statin therapy, most common adverse effect was dyspepsia (10.29%), followed by constipation (5.8%), myalgia, headache and sleep disturbances (2.9%).Mood and behavioural changes were in the form of insomnia, depression, aggressiveness, behaviour change and were seen only in 1 patient (1.4%), while in patients without statin therapy,13% had dyspepsia and 9% complained of constipation and 4.5% of headache. All these adverse effects were clinically insignificant and did not need any change or termination of treatment.
The Statin Therapies for Elevated Lipid Levels compared Across doses of Rosuvastatin (STELLAR) trial randomized 2,431 participants with hypercholesterolemia to treatment with rosuvastatin; simvastalin or pravastatin,all in varying doses. The percentages of patients who reported adverse events during treatment were similar among randomized groups. Adverse events were generally mild and similar across groups. The most common adverse events overall were pain (6%), pharyngitis (5%), myalgia (4%), and headache (3%). Serious adverse events were reported in 29 patients, with the number of serious events ranging from 0 (with rosuvastatin 40 mg) to 5 (simvastatin 40 mg). Changes in clinical laboratory results (increases in liver or muscle enzymes) were generally small.(10)
Similarly, in the lipitor placebo-controlled clinical trial database of 16,066 patients with a median treatment duration of 53 weeks, 9.7% of patients on atorvastatin and 9.5% of the patients on placebo discontinued due to adverse reactions regardless of causality (12); again establishing that the adverse effects in both the groups were comparable. The five most common adverse reactions in patients treated with atorvastatin that led to treatment discontinuation and occurred at a rate greater than placebo were: myalgia (0.7%), diarrhea (0.5%), nausea (0.4%), alanine aminotransferase increase (0.4%), and hepatic enzyme increase (0.4%). The most commonly reported adverse reactions (incidence ≥ 2% and greater than placebo) regardless of causality, in patients treated with atorvastatin in placebo controlled trials (n=8755) were: nasopharyngitis (8.3%),arthralgia (6.9%), diarrhea (6.8%), pain in extremity (6.0%), and urinary tract infection (5.7%).(12)
Other Adverse Reactions Reported In Placebo-Controlled Studies Include malaise, pyrexia; Digestive system: abdominal discomfort, eructation, flatulence, hepatitis, cholestasis; Musculoskeletal system: musculoskeletal pain, muscle fatigue, neck pain, joint swelling; Metabolic and nutritional system: transaminases increase, liver function test abnormal, blood alkaline phosphatase increase, creatine phosphokinase increase. Special senses: vision blurred, tinnitus.The reports are generally nonserious, and reversible upon statin discontinuation.(12)
In ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) study involving 10,305 participants treated with 10 mg atorvastatin daily (n=5,168) or placebo (n=5,137), the safety and tolerability profile of the both the groups was comparable during a median of 3.3 years of follow-up. (12)
In Collaborative Atorvastatin Diabetes Study (CARDS) involving 2,838 subjects with type 2 diabetes treated with atorvastatin 10 mg daily (n=1,428) or placebo (n=1,410), there was no difference in the overall frequency of adverse reactions or serious adverse reactions between the treatment groups during a median follow-up of 3.9 years. No cases of rhabdomyolysis were reported. (13)
Another study, the TNT (Treating to New Targets Study) (14) involving 10,001 with clinically evident CHD treated with atorvastatin 10 mg daily (n=5006) and 80 mg daily (n=4995), there were more serious adverse reactions and discontinuations due to adverse reactions in the high-dose atorvastatin group (92, 1.8%; 497, 9.9%, respectively) as compared to the low-dose group (69, 1.4%; 404, 8.1%, respectively) during a median follow-up of 4.9 years; which correlates to the present study in terms of safety of low dose atorvastatin.
Another study which aimed at statin-associated muscle-related adverse effects,which selected subjects from a subset of patients who participated in the University of California, San Diego (UCSD) Statin Effects Study reported the ADRs as muscle pain (93%), fatigue (88%), and weakness (85%). (15)
Hepatic injury due to statins was reported by a group who analysed episodic reports of adverse drug reactions sent to the Swedish Adverse Drugs Reactions Advisory Committee between 1988 and 2010. They found 73 patients with hepatotoxicity of whom 30 [41%] were taking atorvastatin and 28 [38%] simvastatin with two deaths and one requirement for liver transplant.(16) Previous studies have shown severe adverse hepatic events related to statins to be infrequent. In a post-hoc analysis of the GREACE (Greek Atorvastatin and Coronary Heart Disease Evaluation) Study, seven (<1%) of 880 participants who received a statin discontinued statin treatment because of liver-related adverse effects (transaminase concentrations more than three-times the upper limit of normal).(17) Also,in TNT study persistent transaminase elevations (≥3 – ULN) occurred in 62 (1.3%) individuals with atorvastatin 80 mg and in nine (0.2%) individuals with atorvastatin 10 mg.(14)
In our study, evaluation of hepatic transaminases was done under the heads of no change, mild change (<1.5 times the upper normal limit), moderate change (elevation from 1.5-3 times the UNL) and severe (>3 times the UNL). Results revealed that most of the cases had either no change (72% in group A and 90.9% in Group B) or mild changes (17.6% in group A and 4.5% in group B) to moderate elevations (8.8% in group A and 4.5% in Group B). Also, patients with mild change in hepatic transaminases were excluded because either the change was <1.5 times UNL or on repeated testing it was observed to be normal.
The Acute Liver Failure Study Group reported on 133 prospectively collected cases of acute liver failure due to drug-induced liver injury between 1998 and 2010;only 2 patients [1.5%] had taken atorvastatin. (18) In our study, severe change was observed in 1(1.4%) patient which required termination of therapy comparable to previous study.
Causality assessment done by WHO UMC causality assessment scale established definite (certain) relationship in 12% patients while probable in 23% and 65% ADRs were categorized as possible. Severity assessment recorded 73% of the total ADR as mild, 23% as moderate and only 3.8% as severe. In a similar study of severity assessment of adverse drug reaction and quality of life of dyslipidemia patients on atorvastatin or rosuvastatin versus controlled; done on 60 patients of which 70% were male and 30% were female, it was found that total percentage of adverse drug reaction in group I (treated with atorvastatin)was mild -51.72, moderate 44.8, severe 3.45 and for group II(rosuvastatin) mild (6.89), moderate (86.2), severe (6.89). Also, most frequent adverse drug reaction associated with Atorvastatin was headache, weakness and constipation. (19)
Conclusion
Since statins have become very popular and are being widely prescribed in recent years to lower blood cholesterol and thus find their utility in a spectrum of pathological conditions including hypercholesterolemia, dyslipidaemia, hypertension, diabetes mellitus, coronary artery diseases, etc; the present study was undertaken to study the adverse effects of low dose Atorvastatin in patients with metabolic syndrome. From the present study, it can be concluded that low dose (20 mg/day) atorvastatin treatment is safe, well tolerated and adverse effects are few and mostly mild, transient and reversible emphasizing the fact that benefits of statins greatly outweigh its risks.
But as no drug is without potential for adverse effects, there is need for awareness of risks as well as benefits of all drugs, particularly like statins which have a wide spectrum of indications; and hence calls for further studies of ADRs for early detection, prevention and management of ADRs and reduced morbidity. The understanding of relatively common statin-associated adverse effects will enable clinicians in making decision in choosing appropriate statin in appropriate dose.
Acknowledgements
Authors would like to express their gratitude towards faculty members of department of Cardiology and also to all the patients enrolled in this study for their support and co-operation which enabled the conduction and completion of this study. Authors would like to extend their deep gratitude to Dr Sarabjeet Singh Sir, (DM Clinical Pharmacology) for his unstinting support and encouragement.
Declarations
Funding
none
Conflict of interest
none
Ethical approval
The study was conducted after obtaining approval from the Institutional Ethics Committee.
References
- MedlinePlus: Available at: https://medlineplus.gov/metabolicsyndrome.html accessed 25 october 2016.
- M. Grundy. Metabolic Syndrome : A multiplex cardiovascular risk factor, Journal of Clinical Endocrinology & Metabolism,2007 Vol. 92 (2); 399-404.
- McNamara JR, Campos H, Ordovas JM, Peterson J, Wilson PW, Schaefer EJ. Effect of gender, age, and lipid status on low density lipoprotein sub fraction distribution. Results of the Framingham Offspring Study.Arteriosclerosis1987 sept-oct7(5);483–90. [pubmed].
- Thomas P.Bersot Drug therapy for Hypercholestremia and Dyslipidemia.In:Laurence Brunton Bruce Chabner Bjorn Knollman eds.Goodman and Gilmans’ – The Pharmacological Basis of Therapeutics 12th Edition,McGraw Hill;2011: 893-894.
- Buhaescu I, Izzedine H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin Biochem. 2007;40:575–84. [PubMed].
- Beatrice A. Golomb, M.D ,Marcella A. Evans, Statin Adverse Effects: A Review of the Literature and Evidence for a Mitochondrial Mechanism Am J Cardiovascular Drugs. 2008; 8(6): 373–418.
- Neil J. Stone, Jennifer G. Robinson, Alice H. Lichtenstein, C. Noel Bairey Merz, Conrad et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults ;A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Journal of the American College of Cardiology; july 2014;Vol 63 (25B): 2890-2932.
- National Cholesterol Education Program (NCEP) (2002). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report 106:3143–3421.
- Pasternak RC, Smith Jr, Bairey-Merz CN, et al. Safety of statins. Circulation. 2002; 106: 1024–1028.
- Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol, 2003; 92: 152–160.
- Ramchandran A. Et al : Metabolic syndromne in urban Asian population study using modified ATP-III criteria. Diabetes Res. Clin. Pract. 2003, Jun 60(3): 149-204.
- http://www.rxlist.com/lipitor-side-effects-drug-center.htm accessed 17/02/2017.
- Helen M Colhoun, D John Betteridge, Paul N Durrington, Graham A Hitman,H Andrew W Neil, Michael Mackness et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial,The Lancet,2004,Vol. 364;685–696.
- Waters DD, Guyton JR, Herrington DM, McGowan MP, Wenger NK et al Treating to New Targets (TNT) Study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? Am J Cardiol. 2004 Jan 15; 93(2):154-8.
- Stephanie Cham, B.S., Marcella A. Evans, B.S., Ms. Julie O. Denenberg, M.A., and Dr. Beatrice A. Golomb, M.D., Statin-Associated Muscle-Related Adverse Effects: A Case Series of 354 Patients Pharmacotherapy. 2010 Jun; 30(6): 541–553.
- Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: Reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374–380. pmid:21889469.
- Vasilios G Athyros, MDa, Konstantinos Tziomalos, MDb,Thomas D Gossios, MDc,Theodora Griva, MDa ;Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis, The Lancet,2010 dec Vol 376 :1916–1922.
- Reuben A., Koch DG, Lee WM. Drug-induced acute liver failure: Results of a US multicenter, prospective study. Hepatology. 2010;52:2065–2076 doi: 10.1002/hep.23937. pmid:20949552.
- Shilpa Sah, Dr. Yusra Ahmad and Dr. Puneet Dhameja ; Severity assessment of adverse drug reaction and quality of life of dyslipidemia patients on atorvastatin or rosuvastatin v/s controlled. European Journal of Biomedical AND Pharmaceutical sciences,2016, Vol 3( 6) 441-455.







