Manuscript accepted on :March 03, 2017
Published online on: --
Plagiarism Check: Yes
Shashikumara1, Prathima. C2 and Mohammed Sibgatullah3
1Department of Pharmacology, Chamarajanagar Institute of Medical Sciences, (CIMS).Chamarajanagar.
2Department of Pharmacology, J. S. S Medical College, (A constituent college of JSS University), SS Nagar, Mysore-570015.
3Drug safety physician Vigimedsafe Hyderabad.
Corresponding Author E-mail: sibgatullah2@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1125
Abstract
To evaluate the in vivo antidepressant activity of Ethanolic extract ofAlangium salviifolium (L. f.) Wangerin leaves (EASL) in Swiss albino mice. Ethanolic extract of Alangium salviifolium (L. f.) Wangerin (EASL) leaves were prepared by a continuous method using Soxhlet apparatus. The extract was subjected to phyto-chemical screening followed by acute oral toxicity studies in mice. EASL in the doses of 100 and 200 mg/kg body weight was administered to test groups 1 and 2 respectively. Imipramine hydrochloride 15mg/kg body weight was administered to Standard group by oral route. Test group 3 received 100mg/kg (Per. Oral) of EASL + 10mg/kg (Per.oral) of Imipramine. Control group received Normal saline 10ml/kg body weight. Antidepressant activity was identified by using modified Forced Swimming Test (FST) and Tail Suspension Test (TST). Period of immobility was observed in both the models which was indicative of anti depressant activity. Standard statistical methods were used to evaluate the results. The results showed significant dose dependent antidepressant effect of EASL in Swiss albino mice for both the models in all the test groups (Test group 1, 2 and 3). EASL possess significant antidepressant activity. However, further investigations are required to determine its active constituents and molecular level of target mechanism for further use in humans.
Keywords
Antidepressant; Alangium salviifolium (L. f.) Wangerin; Forced swimming test; Tail suspension test
Download this article as:| Copy the following to cite this article: Shashikumara S, Prathima C, Sibgatullah M. Evaluation of Antidepressant Activity of Ethanolic Extract of Alangium Salviifolium (L. F.) Wangerin in Swiss Albino Mice. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Shashikumara S, Prathima C, Sibgatullah M. Evaluation of Antidepressant Activity of Ethanolic Extract of Alangium Salviifolium (L. F.) Wangerin in Swiss Albino Mice. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13806 |
Introduction
Depression is a type of serious neurological disorder, characterized by disturbances in sleep and appetite as well as deficit in cognition and energy [1]. Depression can be potentially life threatening condition that has affected millions of people across the globe and can occur at any age groups from childhood to later life. It is known to exert a huge burden upon the society. The distinctive symptoms exhibit as a triad forms that include: low or depressed mood, “anhedonia” (reduced ability to experience natural rewards), and low energy or fatigue [2].
Major depressive disorder is a complex and frequent psychiatric condition that poses significant challenges to both the patients who experience it and the physicians who treat them. The goal of therapy is for patients to achieve remission, which requires identifying and measuring symptoms at the outset and throughout treatment to document both response and resistance to treatment. [3]. The life time prevalence of depression is between 10-20% in general population worldwide, with a female to male ratio about 5:2. Typically, the course of the disease is recurrent, and most patients recover from depressive episodes. However, a substantial proportion of patients become chronic and after 5 or 10 years of potential follow up, about 12% and 7% of them respectively are still depressed [4].
Mood disorder are the second primary cause for disability adjusted life years worldwide and the leading cause of years lived with disability in all the age groups in the world. Each drug used to treat this disorder has a success rate of about 60%. In addition, most therapies require several weeks of treatment before improvement of signs and symptoms are observed and there are numerous side effects caused by antidepressants [5].
Alangium salviifolium (L. f.) Wangerin (ankolemara) is one of the most valuable plants in traditional system of the medicine from ancient time..Alangium salviifolium (L. f.) Wangerinis a small shrub or deciduous tree that may or may not be armed. Leaves are alternative, usually unequal, 12.5-17cm long, 2.5-7 .0 cm broad, oblong lanceolate or oblong- oval, acute or rounded, prominent beneath and obtuse at apex with 3-6 pairs of oblique veins with white or yellowish-white colour and fragrance. It is known to contain various phyto-chemicals like alkaloids (ipecac and benzopyridoquinolizidine), flavonoids, triterpinoids, saponins, tannins, phenolic glycosides, volatile oil, alangine, lamarckinine, salviifosides A-C, salicin, kaempferol, and kaempferol 3-O-b-D-glucopyranoside [6].
As per traditional claim, the plant possess different pharmacological activities like anti-cancer, anti-oxidant, anti-bacterial, anti-fungal, anti-inflammatory, and anti-fertility. It is also used in the treatment of anxiety and mood disorders [6]. Therefore, the present study was carried out to evaluate antidepressant activity of ethanolic extract of leaves of Alangium salviifolium (L. f.) Wangerin by stress induced depression by forced swim test model and tail suspension test model in Swiss albino mice.
Material and Methods
The experiment was carried out after obtaining due clearance from Institutional animal ethics committee, 3rd floor, JSS University campus, Mysuru-570015, Karnataka.
Animals
Swiss albino mice weighing 25-30g, of either sex were procured from the central animal facility of the Institute and maintained under the standard conditions: room temperature (25±3) °C, humidity 45%–55%, 12 /12hr light/dark cycle. They were fed with commercially available mouse pellet diet and water was allowed ad libitum.
Before grouping of animals an acute toxicity study was carried out in escalating doses ranging from 50 mg/kg body weight to 2000 mg/kg body weight. The extract was safe in dose up to a dose of 2000 mg/kg body weight. The animals were subjected to wash period of 4 weeks and observed for any toxic effects for up to 48 hours.
Grouping
Animals were randomly divided into 5 groups of 6 each and received drugs as follows:
Group 1: Control group is treated with normal saline (10ml/kg)
Group 2: Standard group treated with drug Imipramine (15mg/kg p.o) [7]
Group 3: Test group-1- ESAL (100mg/kg p.o)
Group 4: Test group -2-ESAL (200mg/kg p.o)
Group 5: -Test group-3-ESAL (100mg/kg p.o) +Imipramine (10mg/kg p.o)
Chemicals
Ethanol, Normal saline, Imipramine (Sun pharmaceuticals)
Plant materials and Preparation of Drug Solution
Alangium salviifolium (L. f.) Wangerin leaves were collected from Bannari Hill (Dimbam), Coimbatore district, Tamilnadu state, and it was authenticated by Dr Mrutyunjaya (Asst. Prof, Dept. of Pharmacognosy, JSS Pharmacy College, Mysore). They were corroborated with the leaves of same plant present in the JSS pharmacy college, Mysore herbarium and preserved for future reference.
The leaves were subjected to wash with 70% of alcohol and made into coarse powder after a shade drying for 1 week. About 500grams of this powder was subjected to soxhlet extraction for 12 hours using ethanol as a solvent under suitable temperature. The extract was further concentrated using vacuum extractor for complete removal of the ethanol.Extract of Alangium salviifolium (L. f.) Wangerin leaves (EASL) was thus obtained and used to evaluate the antidepressant activity in the test animals. Stock solution was freshly prepared by using Normal saline as solvent. Before starting the actual experiment phyto-chemical screening of the ethanolic extract and acute oral toxicity study was carried out.
Forced Swim Test
The forced swim test is a rodent behavioral test used for evaluation of antidepressant drugs, antidepressant efficacy of new compounds, and experimental manipulations that are aimed at rendering or preventing depressive-like states. Mice are placed in an inescapable transparent tank that is filled with water and their escape related mobility behavior is measured. The forced swim test is straightforward to conduct reliably and it requires minimal specialized equipment.
All the groups of animals were subjected to forced swim test after administering the respective drug solutions. On day 0, in training session, mice were forced to swim individually in a vertical Plexiglas cylinder (height: 40 cm; diameter: 18 cm) containing fresh water up to 15 cm maintained at 25°C for 15 minutes and the animals were observed for 6 minutes. In this test, after a brief spell of vigorous activity, animals show a posture of immobility which was characterized by floating motionless in the water making only those movements necessary to keep the head above the water. This immobility reflects the state of depression. Each mouse was subjected to this procedure 24h prior and 1h after administration of respective drugs for 5 minutes in the test session, and the duration of immobility during last 4 minutes was recorded. Actual test recordings were done on 1st, 7th and 14th day of treatment. After recording of mobility-immobility time, the each mouse were removed, wiped with dry cloth and allowed to dry before being returned to their home cages[8, 9].
Tail Suspension Test
The tail-suspension test is a mouse behavioral test useful in the screening of potential antidepressant drugs, and assessing of other manipulations that are expected to affect depression related behaviors. Mice are suspended by their tails with tape, in such a position that it cannot escape or hold on to nearby surfaces. During this test, typically six minutes in duration, the resulting escape oriented behaviors are quantified. The tail-suspension test is a valuable tool in drug discovery for high-throughput screening of prospective antidepressant compounds.
All the groups of animals were subjected to this test by suspending them on a string held by a metal stand, by an adhesive tape placed 1 cm from the tip of the tail and the string was 58 cm above the table top. The duration of mobility- immobility of the mice was recorded for a period of last 4 minutes during a period of 5 minutes observation. Mice were considered immobile when they hang passively and completely motionless. During the experiment, each animal under test was both acoustically and visually isolated from other animals. Mice were considered immobile when they hang passively and completely motionless. Readings were taken on 1st, 7th and 14th day of treatment.
Statistical Analysis
The results were computed using GRAPH PRISM PAD version 7 software.One way ANOVA test followed by Post-hoc Tukey’s multiple comparison tests were applied for analysis. Observations were expressed as mean ±SD/SEM. The differences between means were considered to be significant at p<0.05(95% confidence individuals)
Results
Phytochemical Screening Test
The freshly prepared extract was subjected to phyto-chemical screening tests for the detection of various active constituents. The extract showed the presence of alkaloids, tannins, steroids, phenolic and flavonoids, carbohydrates, and glycosides in crude extract of Alangium salviifolium (L. f.) Wangerin leaves as depicted in Table 1.
Table 1: Result of chemical group tests of the Ethanolic extract of Alangium salvifolium leaves.
| Extract | Carbohydrates | Tannins | Flavonoid | Saponin | Phenols | Steroids | Alkaloids | Glycosides |
| Alangium salviifolium (L. f.) Wangerin | ++ | ++ | ++ | – | ++ | + | +++ | +++ |
EE- Ethanolic extract; (+): Present; (-): Absent; (+++); Reaction intensity is high; (++): Reaction intensity is medium; (+): Reaction intensity is normal;
Acute oral Toxicity Study
The acute toxicity study aims in establishing the therapeutic index, i.e. the ratio between the pharmacologically effective dose and lethal dose on the same strain and species. The extract of was safe up to the dose of 2000mg/kg (Per oral) body weight. Behaviour of the animals was closely observed for the first 3 h then at an interval of every 4 h during the next 48h. The extract did not cause mortality in the mice during 48h observation. There was no significant difference in food and water intake among the animal groups studied. Then the results of the LD50 study performed on mice were expressed using Karber’s method. The results obtained were expressed in the table no. 2. From the table no. 2, it can be concluded that there is no mortality and toxicity symptoms for the ethanolic extract. So the dose was optimized up to 2000mg/kg [11]. (Table 2).
Table 2: Shows Acute Toxicity Studies on Ethanolic Extract of AlangiumSalvifolium leaves.
| Group | Dose(mg/kg) | No of animals | Dose differences (a) | Mortality (b) |
| 1 | 50 | 6 | No | |
| 2 | 100 | 6 | 50 | No |
| 3 | 500 | 6 | 400 | No |
| 4 | 1000 | 6 | 500 | No |
| 5 | 1500 | 6 | 500 | No |
| 6 | 2000 | 6 | 500 | No |
LD50 = higher dose -Σ (a x b) /n
n = No. of animals in each group
LD50 = 2000 – 0
= 2000 mg / kg
ED50 = LD50/ 10
= 2000 / 10
= 200 mg / kg.
Forced Swim Test
The results of acute model of FST with mice are displayed in Table-3 & Graph-1. In this test, animals of all the test groups showed significant results.The EASL extract (100 & 200mg/kg body weight) treated groups exhibited significant delay in the onset of immobility and also significantly reduced time of immobility in the forced swimming test after 14 day of treatment. Post-hoc Tukey’s multiple comparison tests analysis demonstrated that the test treatment significantly reduced the immobility time in comparison to the control group (p<0.0001). Combination of EASL extract (100mg/kg b wt) and imipramine in the reduced dose (10mg/kg b wt.) i.e. Test group-3 showed significantly reduced immobility and increase in the normal behaviour of mice in water filled apparatus and also exhibited antidepressant activity comparable to the standard drug Imipramine (10mg/kg b wt.) i.e. the standard group. On day-1 of the test the immobility time was 144.2± 0.94, 135.2± 1.07, 98.6± 2.49 seconds in test groups 1, 2 and 3. The results were statistically significant in test groups 2 and 3 when compared to control in which the immobility time was 158.5± 3.87 seconds. However with subsequent drug administration the immobility time was significantly reduced in all test groups to 136.7± 1.45, 130.7± 0.8 and 95.67± 4.41 seconds (test groups 1, 2 and 3 respectively) when compared to control group which was 152.7± 3.89 seconds on day 7 of the test. On day 14 also reduction in immobility time was significant in all test groups at 135.7± 1.7, 127.8± 0.4 and 83.67± 0.42 seconds (test groups 1, 2 and 3 respectively) when compared to the control group which was 148.5± 3.73 seconds. However, the results of the standard drugs were significantly better on all the test days at 96.5± 9.0, 80± 4.46 and 72.5± 3.72 seconds on day 1, 7 and 14 respectively.
Table 3: Effects of EASL on immobility time in mouse forced swimming test (FST)
| Group no. | Treatment | Immobility Time (s) | ||
| Day 1 | Day 7 | Day 14 | ||
| I | Vehicle Control (10ml/kg) | 158.5±3.87 | 152.7±3.89 | 148.5±3.73 |
| II | Imipramine (15mg/kg) | 96.5± 9.0∗∗∗∗ | 80±4.46∗∗∗∗ | 72.53.72∗∗∗∗ |
| III | EASL (100mg/kg) | 144.2±0.94ns | 136.7±1.45∗∗∗ | 135.7±1.70∗∗∗ |
| IV | EASL (200 mg/kg) | 135.2±1.07∗∗∗∗ | 130.7±0.80∗∗∗∗ | 127.8±0.40∗∗∗∗ |
| V | EASL +Imipramine(100mg/kg + 10mg/kg) | 121.6±2.49∗∗∗∗ | 129.67±4.41∗∗∗∗ | 109.67±0.42∗∗∗∗ |
Values are expressed as mean ± SEM. Comparison between control v/s all the other groups. Statistical test done by one-way ANOVA followed by Post-hoc Tukey’s multiple comparison test, *p<0.05, **p<0.01; *** p<0.001; ****p<0.0001.
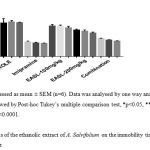 |
Figure 1: Effects of the ethanolic extract of A. Salvifolium on the immobility time in the mouse Forced swim test |
Effect of EASL on immobility in the FST using mice
Tail Suspension Test
The results of the antidepressant effect of Ethanolic extract of Alangium salviifolium (L. f.) Wangerin are presented in Table-4 and figure-2. The extract showed slight reduction in immobility on 1 day treatment, but significantly reduced the immobility time after 7 and 14 days of treatment. Combined group showed almost nearly same significant reduction in immobility time comparable to standard Imipramine. On day 1 of the test the immobility time in test groups 1, 2 and 3 was 232.3± 5.39, 201.7± 3.45 and 135.8± 1.815 seconds respectively which was statistically significant when compared to the control group in which the immobility time was 257± 13.92 seconds. On day 7 the of the test the immobility time in test groups 1, 2 and 3 was 234.8± 2.91, 194.8± 3.96 and 126.7± 3.59 seconds respectively which was statistically significant when compared to the control group in which the immobility time was 264± 4.43 seconds. Similarly on day 14 of the test the immobility time in test groups 1, 2 and 3 was 218.8± 4.92, 178.3± 3.59 and 116.5± 1.61 seconds respectively which was statistically significant when compared to the control group in which the immobility time was 257± 2.15 seconds. The standard drug was far superior in reducing the immobility time on all days of the test at 112.3± 2.52, 103.8± 2.04 and 100± 1.155 seconds respectively on days 1, 7 and 14 of the test.
Table 4: Effects of EASL on immobility time in mouse Tail suspension test (TST)
| Group no. | Treatment | Immobility Time (s) | ||
| Day 1 | Day 7 | Day 14 | ||
| I | Vehicle Control (10ml/kg) | 257±13.91 | 264.8.5±4.43 | 257.8±2.15 |
| II | Imipramine (15mg/kg) | 112.3±2.525∗∗∗∗ | 103.8±2.04∗∗∗∗ | 1001.155 |
| III | EASL (100mg/kg) | 232 .3±5.391∗∗ | 234.8±2.921∗ | 218.8 ±4.922∗∗∗∗ |
| IV | EASL (200 mg/kg) | 201.7±3.451∗∗∗∗ | 194.2±3.962∗∗∗∗ | 178.3± 3.593∗∗∗∗ |
| V | EASL +Imipramine (100mg/kg + 10mg/kg) | 135.8±1.815∗∗∗∗ | 126.7± 0.9888∗∗∗∗ | 116.5±1.607∗∗∗∗ |
Values are expressed as mean ± SEM. Comparison between control v/s all the other groups. Statistical test done by one-way ANOVA followed by Post-hoc Tukey’s multiple comparison test, *p<0.05, **p<0.01; *** p<0.001; ****p<0.0001.
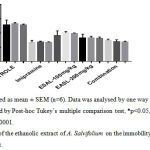 |
Figure 2: Effects of the ethanolic extract of A. Salvifolium on the immobility time in the mouse Tail suspension test.
|
Effect of EASL on immobility in the TST using mice
Discussion
The present study revealed the significant anti-depressant effect of ethanolic extract of Alangium salviifolium (L. f.) Wangerin leaves in experimentally induced depression by Forced swim test and Tail suspension test models. The ethanolic extract of Alangium salviifolium (L. f.) Wangerin leaves significantly decreased the immobility time in dose dependent manner which is an indicator of antidepressant activity.
Alangium salviifolium (L. f.) Wangerinis known to contain natural phytonutrients such as alkaloids (ipecac and benzopyridoquinolizidine), Glycosides, Steroids, flavonoids, Amino acids which may be responsible for improving the vital neurotransmitters involved in memorization, information and processing that may be helpful in depression. Literature review of the plant reveals that Alangium salviifolium (L. f.) Wangerin also contains Flavonoids & Tannin [13]. Different types of neuroactive steroids were found to be ligands for the GABA receptors in the central nervous system; which indicates that they act as a benzodiazepine like molecules [14].
The anti-depressant effect may be attributed to the active compounds in the extract that act on GABA/benzodiazepine receptor complex as well as by stimulating glucocorticoid production and its release in the adrenal cortex [11.12].
EASL extract reduced the immobility period during the forced swimming and tail suspension test in comparison with control and exhibited a dose dependent antidepressant activity. The characteristic behaviour evaluated in these test, termed immobility, has been considered to reflect behavioural despair similar to that seen in the human depression, and hence any reduction in this parameter reflects antidepressant activity. There is a significant correlation between the clinical efficacy of antidepressant drugs and their potency in FST which was not found in any other model. Interestingly, our data indicate that higher doses of plant extracts were more effective than smaller doses both in forced swim test and tail suspension tests.
The major inhibitory neurotransmitter in central nervous system is Gamma amino butyric acid (GABA). Different type of antidepressants, muscle relaxants, sedative- hypnotic drugs exhibit their action through GABA-ergic inhibition in the CNS that leads to either decrease in the firing rates of critical neurons in the brain or direct activation of GABA receptors by the extracts [14]. This result indicates the significantly decreased immobility period in FST and TST by EASL. A probable mechanism being our plant extracts acting through GABAergic and/or glutamatergic transmission, cytokine or steroidal alterations cannot be ruled out.
Though the EASL extract have a modest effect when compared to standard it can serve as an add-on drug to current regimens or may be used along with current regimens in lower dose. The reduction in dose of these Standard drugs is always a welcome change and may help in reducing the adverse effect profile which becomes obvious at higher doses. Further isolation and identification of the bioactive ingredient responsible for anti depressant activity is necessary.
Conclusion
The present study has showed antidepressant activity of EASL in all classic models such as forced swimming test (FST) and tail suspension test (TST) comparable to the standard drug Imipramine hydrochloride. However, further studies are needed to elicit its exact mechanism of action and to identify the active ingredient as a potent and efficacious antidepressant agent.
Acknowledgement
There is no conflict of interest. The authors acknowledge the entire staff of Department of Pharmacology, JSS Medical College, Mysuru-15.
References
- Porter RJ, Meldrum BS, Charles De Battista, Katzung BG, Masters SB, Trevor AJ. Antidepressant Agents. Basic and clinical Pharmacology. 11th Edition. New Delhi: Tata McGraw Hill; 2009: 521 -525.
- Frasure-Smith N. and Lesperance F.: Depression and coronary artery disease. Herz 2006; 31: 64-68.
- Culpepper, Larry et al. Major Depressive Disorder: Understanding the significance of residual symptoms and balancing efficacy with tolerability. The American Journal of Medicine 2015; 128(9): S1 – S15.
- Keller M.B., Hirschfeld R.M., and Hanks D.: Double depression: A distinctive subtype of unipolar depression. J Affect Disord 1997; 45: 65-73.
- Ashoka kumar, B.S, Lakshman, K, Velmurgan, C, Sridhar, S.M, and Gopisetty Saran. Antidepressant activity of methanolic extract of Amaranthus spinous. Winter2004; 5(1):11-17.
- Ravirala Venkateshwarlu, Dr. Akondi Butchi Raju, Venu Gopal Yerragunta.St. Peter’s institute of pharmaceutical sciences, Hanmakonda, Waranga. “Phytochemistry and pharmacology of Alangium salviifolium (L. f.) Wangerin: A review”. Journal of Pharmacy Research 2011; 4(5): 1423-1425.
- Dhingra D, Sharma A. Antidepressant-like activity of Glycyrrhiza glabra. Prog. Neuropsychopharmacol. Biol. Psych. 2006; (30):449-454.
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The Mouse Forced Swim Test.Journal of Visualized Experiments : JoVE. 2012;(59):3638.
- Porsolt RD, Bertin A, Jalfre M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch Int Pharmacodyn Ther. 1977; 229:327-336
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The Tail Suspension Test.Journal of Visualized Experiments : JoVE. 2012;(59):3769.
- Maridassa, Hussain MIZ, and Rajuc G. Phytochemical Survey of Orchids in the Tirunelvies Hills of South India. Ethnobotonical Leaflets 2008:12:705(6):798-804.
- Khatum M H, Islam MR, Mamun A. Nahar L, Luthfunnesa, and Islam M. A. U. In Vivo Evaluation of Hibiscus sabdarifffa Fruits’ J of ppli Sciec Rese 2011; 7(6):798-804.
- Nishikava H, Hata T, Funakami Y. Arole for corticostropin- releasing factor in repeated cold stress- induced anxiety- like behavior during forced swimming and elevated plus maze test in mice. Biol Pharma Bull 2004 ;( 3):352-356.
- Millan MJ, Hjorth S, Samania R, Schreibr R, Jaffard R, DeLadonchamps B S15535, A novel benzodioxopiperazine ligand of serotonin (% 5HT) 1A receptor:II. Modulation of hippocample serotonin release in relaxation of potential anxiolytic properties. J Pharmacol Exp Ther 1997; 282:148-161.







