Manuscript accepted on :14-02-2024
Published online on: 07-05-2024
Plagiarism Check: Yes
Reviewed by: Dr. Durgeshranjan Kar and Dr. Shiv Gunjegaonkar
Second Review by: Dr. Sohayla Mohamed Elsherbini Attalla
Final Approval by: Dr Jihan Seid Hussain
Maximus M. Taek1* , Burhan Ma’arif2
, Burhan Ma’arif2 , Faisal A. Muslikh
, Faisal A. Muslikh , Novia Maulina2
, Novia Maulina2  and Paulus R. F. Lalong4
and Paulus R. F. Lalong4
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Widya Mandira Catholic University, Kupang, Indonesia.
2Department of Pharmacy, Faculty of Medical and Health Science, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia.
3Department of Pharmacy, Faculty of Pharmacy, Bhakti Wiyata Health Sciences Institute, Kediri, Indonesia.
4Department of Biology, Faculty of Mathematics and Natural Sciences, Widya Mandira Catholic University, Kupang, Indonesia.
Corresponding Author E-mail: maximusmt2012@unwira.ac.id
Abstract
Malaria is a global health concern that threatens many countries. Plasmodium sp. may facilitate human transmission and is currently relatively resistant to chloroquine. The people in Nusa Tenggara, Indonesia, have traditionally employed the cortex of Black Pulai (Alstonia spectabilis) as a treatment for malaria for a considerable period. The objective of this study is to analyze the phytochemical composition of the 96% ethanol extract of A. spectabilis cortex (ASCE) and evaluate the antimalarial properties of tablets derived from A. spectabilis cortex extract (ASCT). A metabolite profile analysis was conducted on ASCE utilizing a UPLC-QToF-MS/MS method. And antimalarial test was conducted on ASCT on mice (Mus musculus) infected with Plasmodium berghei, and the blood smears of the mice were analyzed along with liver tissue damage. The results showed that administering ASCT could reduce the percentage of parasitemia in mice as well as the average liver damage score. This situation is feasible due to the presence of significant compounds in the ASCE that are anticipated to function as antiplasmodium agents, including villalstonine, vincadifformine, and pleiocarpamine. From these findings, one can infer that ASCT, which includes ASCE as its active component, has the potential to serve as a preferred antimalarial medication.
Keywords
Alstonia Spectabilis; Antimalarial; Metabolite Profiling; Tablet
| Copy the following to cite this article: Taek M. M, Ma’arif B, Muslikh F. A, Maulina N, Lalong P. R. F. Metabolite Profiling of the Extract and Antimalarial Activity of the Tablets Derived from the Cortex of Alstonia spectabilis. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Taek M. M, Ma’arif B, Muslikh F. A, Maulina N, Lalong P. R. F. Metabolite Profiling of the Extract and Antimalarial Activity of the Tablets Derived from the Cortex of Alstonia spectabilis. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3ybhwLC |
Introduction
Malaria is a global health problem that threatens lives in more than 104 countries 1 and it is currently estimated that there are 300-500 million cases each year 2,3. Various Plasmodium species, such as Plasmodium vivax, Plasmodium falciparum, Plasmodium malariae, and Plasmodium ovale 4, are capable of causing this human transmission. Malaria cases decreased from 238 million to 229 million in 87 malaria-endemic countries, according to the World Health Organization (WHO), with cases per 1000 population at risk falling from 80 in 2000 to 57 in 2019 5,6. In Indonesia, this disease is a very serious problem, this is proven by the high morbidity rate and causing many deaths 7. This condition is because Indonesia has a heterogeneous climate and is vulnerable to regional and global climate change, thereby increasing the risk of transmission via mosquito vectors 8.
This disease really threatens people’s health, especially for poor people living in remote areas, besides that it can also reduce productivity, cause economic losses, and increase the mortality of babies, children and adults 4. The WHO characterizes malaria elimination as the cessation of local transmission, signifying a reduction in the occurrence of malaria cases to zero in specific geographical regions 3. However, over the past few decades, there has been a growing resistance of Plasmodium parasites to antimalarial quinine derivatives, particularly chloroquine, rendering the drug ineffective 9.
The increasing resistance of Plasmodium to the chloroquine, and there are also side effects of chloroquine such as gastrointestinal disorders, headaches, blurred vision, insomnia, and exacerbation of psoriasis 10, encourages researchers to continue looking for new and more effective antimalarial drugs 11. Plants have become one of the most important sources in the search for potential new antimalarial drugs in the traditional medicine of various ethnicities throughout the world 12. The indigenous people of Nusa Tenggara, Indonesia, traditionally employ Black Pulai (Alstonia spectabilis) as an antimalarial remedy 13, This plant has demonstrated robust inhibitory activity against the P. falciparum 3D7 strain, as confirmed through in vitro testing in initial research 9.
The great benefits contained in A. spectabilis have great potential for development to be used as a medicinal product so that it is more easily accepted by patients, but the difficulty of penetrating active substances from natural ingredients into the body due to differences in lipid solubility and molecular size is an inhibiting factor for drug molecules to pass through biological membranes thereby interfering with systemic absorption 14. One solution to form a dosage form that has good stability is tablets 15, therefore further investigation has been conducted to formulate tablets using a 96% ethanol extract derived from the cortex of A. spectabilis.
This study aims to assess the phytochemical composition of A. spectabilis cortex extract (ASCE) utilizing ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QToF-MS/MS). Additionally, it seeks to evaluate the antimalarial properties of tablets derived from A. spectabilis cortex extract by conducting experiments on mice infected with P. berghei.
Materials and Methods
Materials
Plant material
A. spectabilis cortex was obtained from the Nusa Tenggara area of Indonesia and has been determined at the UPT Materia Medica, Batu, Indonesia.
Chemical material
Merck (Darmstadt, Germany) supplied ethanol 96%, formic acid, dichloromethane, methanol, and wright eosin, while Smart-Lab Indonesia (Tangerang, Indonesia) provided acetonitrile H-1010. Imedco (Tangerang, Indonesia) was the source for ket-A-100, xylazine 2%, and hydroxychloroquine sulfate.
ASCT
ASCT was formulated and provided to us by PT. Agaricus Sido Makmur Sentosa, Malang, Indonesia). ASCT was formulated using wet granulation, with the active ingredient being 96% ethanol extract of A. spectabilis cortex. Each ASCT is equivalent to 100 g of simplicia. The excipients used as constituents of the tablet formula were lactose, microcrystalline cellulose, sodium starch glycolate, silica hydrate, magnesium stearate, copovidine, and methylparaben.
Parasite culture
The P. berghei culture used is the ANKA strain, which was obtained from the Health Animal Clinic Research and Diagnostic Laboratory, Malang, Indonesia.
Animal subject
The test animals used were male Swiss mice (Mus musculus) weighing 20-25 g and 2-3 months old. These mice were obtained from the Biosciences Institute, Brawijaya University and have passed the ethical suitability test from the Brawijaya University Research Ethics Commission with ethical suitability letter No: 156-KEP-UB-2023.
Method
Extraction
Roughly 500 g of powdered A. spectabilis cortex underwent ultrasonic extraction with 96% ethanol through three ten-minute cycles, utilizing a Soltec Sonica 5300EP S3. The resulting solution was filtered and dried at 50 °C using a Heidolph G3 rotary evaporator. After this procedure, 25 g of the 96% ethanolic extract was acquired and subsequently subjected to additional testing and analysis, in this study, metabolite profiling was carried out 16.
Metabolite profiling
Metabolite profiling was conducted using a UPLC[JSHS9] [BM10] -QToF-MS/MS instrument at the Forensic Laboratory Center of the Indonesian National Police Criminal Investigation Agency. A 96% ethanolic extract was generated through solid-phase extraction (SPE) using dichloromethane and methanol as solvents. Subsequently, 5 L of the extract was evenly injected into an ACQUITY UPLC® H-Class System (Waters, USA) equipped with an MS Xevo G2-S QToF detector (Waters, USA). The separation of samples occurred on an ACQUITY BEH C18 column (1.7 μm × 2.1 mm × 50 mm) with acetonitrile + water and 0.05% formic acid + 0.05% formic acid as mobile phases. The flow rate was maintained at 0.2 mL/minute. MassLynx 4.1 was employed to process the UPLC-QToF-MS/MS analysis results, generating chromatogram data and m/z spectra for each detected peak. The identified compounds were then validated using online databases, including MassBank (https://massbank.eu/MassBank), ChemSpider (http://www.chemspider.com), and PubChem (https://pubchem.ncbi.nlm.nih.gov).
Antimalarial activity test
The procedure for testing the antimalarial activity of ASCT followed the standard Peter’s Test Method 17. This research used a completely randomized design (CRD) through an experiment with four treatment groups, where each treatment consisted of three mice. Normal control group (without any induction), negative control group (mice induced by P. berghei), positive control group (mice induced by P. berghei + hydroxychloroquine sulfate 0.2 mg/20 gBW mice/day), and treatment group (mice induced by P. berghei + ASCT at a dose of 5.46 mg/20 gBW of mice/day).
Mice were acclimatized for 7 days before the P. berghei inoculation process. Inoculation was carried out by administering 1×107/0.1 ml of suspension intraperitoneally to donor mice that had a parasitemia percentage of more than 20%. Donor mice blood was taken and infected as much as 1×107/0.1 ml into treated mice intraperitoneally.
Antimalarial activity test on mice according to treatment groups. Treatment is given if all test mice show parasite growth with parasitemia ± 1% after 24 h post-infection. The test material was administered using a gastric probe once a day for 3 days.
The observation of parasitemia rates was carried out for 7 days to evaluate the effectiveness of ASCT. Thin blood smear preparations are made by dripping blood onto an object glass, then staining with Morphology of Peripheral Blood (MDT) dye. Meanwhile, liver tissue observation was carried out using liver damage scoring, necrosis, and degeneration. Blood and liver preparations were scrutinized using a light microscope (Nikon Eclipse type Ei) assisted by an Optilab microscope camera. The examination involved observing 10 different fields of view at a magnification of 1000 times for blood preparations. In the case of liver preparations, the examination was conducted in 5 fields of view with a magnification of 400 times.
Results and Discussion
Metabolite profiling
In order to determine the specific compounds responsible for the antimalarial activity of ASCE, the UPLC-QToF-MS/MS instrument was utilized to identify secondary metabolite compounds. The total ion chromatogram (TIC) is visible in (Figure 1). In the interim, the percent area, m/z, retention time (RT), compound name and molecule formula values are displayed in (Table 1).
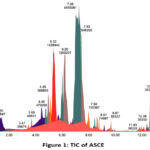 |
Figure 1: TIC of ASCE
|
 |
Table 1: Prediction of compounds in ASCE
|
Following the metabolite profiling conducted through UPLC-QToF-MS/MS, accelerated solvent extraction produced a collection of 37 compounds. Among these, 8 were unidentified, and 29 were recognized or known compounds. In the process of metabolite profiling, not every peak in TIC can be identified among all compounds that have been detected. An indication of this is the presence of unknown compounds, which are those that are not included in the database. These compounds may consist of undetected impurities or degradants by the instrument, as well as novel compounds that have not yet been added to the database. The unidentified compounds with elevated concentrations are of particular significance. Several “major” or “dominant” compounds are identified through the examination of these metabolite compounds 18,19. The percent area indicates that these compounds are more abundant in the sample compared to other compounds. In the ASCE, the major compounds were the alkaloid villalstonine (44.6101 %), vincadifformine (22.2509 %), and pleiocarpamine (10.2855). Every known major compound possesses antimalarial properties.
Antimalarial activity test
The analysis of parasitemia percentage revealed the growth of P. berghei, which was inoculated into mice, commencing on day 1 (D1). The assessment of parasite growth involved calculating the number of erythrocytes infected with ring, trophozoite, and schizont stages per thousand erythrocytes, multiplied by 100%. Table 2 presents the results of parasitemia growth before any treatment. It’s noteworthy that malaria is transmitted by the hemoprotozoa P. berghei to small rodents. As an anti-malarial drug screening model, P. berghei is extensively utilized, owing to its status as a malaria parasite that exclusively infects mammals apart from humans. The in vivo evaluation of this parasite was justified on the grounds that its structure, physiology, and life cycle are remarkably similar to those of human malaria 20.
Table 2: % growth of parasitemia
|
% parasitemia growth |
||
|
Group |
Before treatment |
After treatment |
|
Normal Control |
0.00 |
0% |
|
Negative Control |
90.00 |
26% |
|
Positive Control (Chloroquine 0.2 mg/20 gBW mice/day) |
92.00 |
15% |
|
ASCT 5.46 mg/20 gBW of mice/day |
95.00 |
14% |
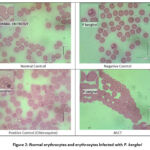 |
Figure 2: Normal erythrocytes and erythrocytes infected with P. berghei
|
In Table 2, it is presented that the growth of parasites in the ASCT group showed that ASCT had good results in inhibiting the growth of parasitemia compared to the negative control and the chloroquine groups. In Figure 2, illustrates the distinctions in the features of regular and infected erythrocytes. Normal erythrocytes exhibit a yellowish hue and lack a nucleus. In contrast, erythrocytes infected with P. berghei appear paler, dotted, and larger in size compared to normal erythrocytes. 21.
Table 3: Average Liver Damage Scoring
|
Average Liver Damage Scoring |
||
|
Group |
Necrosis |
Degeneration |
|
Normal Control |
0.7 |
0.8 |
|
Negative Control |
4.0 |
3.8 |
|
Positive Control (Chloroquine 0.2 mg/20 gBW mice/day) |
1.3 |
2.0 |
|
ASCT 5.46 mg/20 gBW of mice/day |
2.5 |
2.1 |
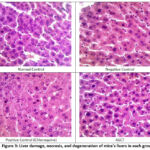 |
Figure 3: Liver damage, necrosis, and degeneration of mice’s livers in each group
|
The results of Table 3 and Figure 3 show that the ASCT group gave a lower liver damage scoring value compared to the negative group, both from necrosis and degeneration scoring. Degeneration is an early sign of liver damage due to toxins, which is temporary (reversible), and cells can still recover or return to normal if toxin exposure is stopped. Degeneration is distinguished by alterations in the cell cytoplasm, marked by an augmentation in cell fluid and swelling. However, the cell nucleus can maintain its integrity as long as the cell does not undergo severe injury 22. This degeneration occurs due to oxidation disorders, which cause the buildup of protein deposits in the cytoplasm, so that the cytoplasm appears cloudy. Parenchymatous degeneration represents the least severe form of degeneration and is considered reversible. Continued parenchymatous degeneration can cause hydrophic degeneration. Hepatocytes that experience hydrophilic degeneration will swell and appear as vacuoles filled with water in the cytoplasm. The cause of persistent damage to hepatocytes can be tearing of the plasma membrane and changes in the nucleus, so that hepatocytes experience necrosis. In addition, necrosis can occur as a result of reactive chemical metabolites binding to nucleophilic proteins within hepatocytes or the presence of metabolites with free chains capable of covalently binding to proteins and unsaturated fatty acids in cell membranes. This process leads to lipid peroxidation and membrane damage, ultimately culminating in hepatocyte death 23.
The results of blood observations and liver damage showed that the ASCT group with dose 5.46 mg/20gBW of mice/day could be an alternative herbal antimalarial treatment. In this study, it was proven that ASCT had the ability to inhibit the growth of the P. berghei parasite in the blood of mice better than the positive control chloroquine. This is thought to be the way antimalarial drugs work in inhibiting the growth of parasites in the blood of mice so that they can reduce the percentage of parasitemia during the three days of observation.
The ASCE contains several antimalarial compounds, one of which is alkaloids, where alkaloid compounds inhibit parasite growth by blocking parasite growth through intracellular transport of choline. In addition to alkaloid compounds as antimalarials, namely by inhibiting the detoxification process of parasite hemorrhoids through vacuoles, food becomes a non-toxic malaria pigment 24.
Villalstonine is one of the compounds contained in ASCE. This compound has demonstrated diverse biological activities, encompassing anticancer, antiamoebic, and antimalarial properties. In antimalarial potential, villalstonine has 1/15th potency compared to chloroquine as single compound in fighting malaria and strong antiplasmodial activity against the multidrug-resistant K1 strain of P. falciparum with an IC50 value of 0.27 μM 25, 26. Vincadifformine and Pleiocarpamine are also compounds found in ASCE. Vincadifformine is recognized for its antimalarial biological activity against P. falciparum, exhibiting efficacy on the A FcM strain (a chloroquine-resistant strain with an IC50 for chloroquine of approximately 230 ng/mL) and the Nigerian strain (a chloroquine-sensitive strain with a chloroquine IC50 of about 41 ng/mL) 27. In contrast, the compound Pleiocarpamine demonstrates antimalarial activity as evidenced by test results utilizing an antiplasmodial assay with the induction of P. falciparum strain K1 and in vivo testing involving mice induced by P. berghei 28.
Conclusion
Based on these findings, it is possible to conclude that ASCT with ASCE as an active ingredient has an antimalarial effect by lowering the proportion of parasitemia in mice as well as the average liver damage score. In ASCE, several key chemicals, including villalstonine, vincadifformine, and pleiocarpamine, are predicted to be responsible for this effect.
Acknowledgment
The authors express their gratitude to PT. Agaricus Sido Makmur Sentosa for providing the ASCT in this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
This research was supported and funded by Pendanaan Program Bantuan Biaya Luaran Prototipe 2023 from Directorate General of Higher Education, Research and Technology Republic of Indonesia number 0921/E5.5/AL.04/2023.
References
- Tabernero, Patricia; Fernández, Facundo M; Green, Michael; Guerin, Philippe J; Newton, Paul N. Mind The Gaps – The Epidemiology Of Poor-Quality Anti-Malarials In The Malarious World – Analysis Of The Worldwide Antimalarial Resistance Network Database. Malaria Journal., 2014; 13(1). doi:10.1186/1475-2875-13-139
CrossRef - Merici, A., Fitri, LE, & Budiarti, N. The Effect of Artesunate and Brotowali (Tinospora Crispa) Combination on Histopathological, and Expression of Nuclear Factor Kappa B (NF-kβ) in Renal Tubules of Mice Infected With Plasmodium Berghei., Clinical and Research Journal in Internal Medicine., 2014: 1 (1). doi:10.21776/ub.crjim.2020.001.01.5
CrossRef - Mayasari, H., Windusari, Y., & Hasyim, H. Malaria Elimination in Various Countries: Literature Review. Journal of Nursing and Public Health, 2023; 11 (1), 73-83. doi: 10.37676/jnph.v11i1.4086
CrossRef - Avichena, A., & Anggriyani, R. Analysis of Malaria Disease Caused by Plasmodium sp Against Human Blood. ECOTONIA: Research Journal of Biology, Botany, Zoology and Microbiology., 2023; 8 (1), 30-37.
CrossRef - Liu, Q., Jing, W., Kang, L., Liu, J., & Liu, M. Trends Of The Global, Regional And National Incidence Of Malaria In 204 Countries From 1990 To 2019 And Implications For Malaria Prevention. Journal of Travel Medicine., 2021; 28 (5), taab046.
CrossRef - Kencanawati, DAPM, Mirong, ID, & Martha, E. A Systematic Scoping Review Of Malaria Prevention Programs In Pregnancy. Journal of Health Sciences., 2022; 3 (12), 1777-1784. doi: doi.org/10.46799/jhs.v3i12.791
CrossRef - Setiyabudi, R. Systematic Review Of Risk Factors For Malaria As An Infectious Disease In Indonesia. MEDICAINS: Scientific Journal of Health Sciences., 2016; 14 (1).
- Tya Palpera Utami, Hamzah Hasyim, Ummi Klatsum, Uthu Dwifitri, Yanti Meriwati, Yuniwarti, Yusro Paridah, Zulaiha. Risk Factors Causing Malaria in Indonesia: Literature Review. Surya Medika Journal (JSM)., 2022; 7(2), 96-107. doi: doi.org/10.33084/jsm.v7i2.3211
CrossRef - Taek MM, Tukan GD, Prajogo BEW, Agil M. Antiplasmodial Activity and Phytochemical Constituents of Selected Antimalarial Plants Used by Native People in West Timor Indonesia. Turk J Pharm Sci., 2021 Feb; 18(1): 80–90. doi:doi.org/10.4274%2Ftjps.galenos.2019.29000
CrossRef - Sepulveda, N. A., Jenkins, J. D., Edington, A., Mallapragada, D. S., & Lester, R. K. The design space for long-duration energy storage in decarbonized power systems. Nature Energy., 2021; 6(5), 506-516. doi: 10.1038/S41560-021-00796-8
CrossRef - Akuodor GC, Ajoku GA, Ezeunala MN, Chilaka KC, Asika EC. Antimalarial potential of the ethanolic leaf extract of Pseudocedrala kotschyi. J Acute Dis., 2015;14:23 –27. doi: 10.1016/S2221-6189(14)60077-9
CrossRef - Shaffer, D., Scott, M., Wilcox, H., Maslow, C., Hicks, R., Lucas, C. P., … & Greenwald, S. The Columbia SuicideScreen: Validity and reliability of a screen for youth suicide and depression. Journal of the American Academy of Child & Adolescent Psychiatry, 2004; 43(1), 71-79. doi: 0.1097/00004583-200401000-00016.
CrossRef - Taek MM, Banilodu L, Neonbasu G, Watu YV, Prajogo BEW, Agil M. Ethnomedicine of Tetun ethnic people in West Timor Indonesia: philosophy and practice in the treatment of malaria. Integrative Medicine Research., 2019; 8(3), 139-144. doi: 10.1016/j.imr.2019.05.005
CrossRef - Ajazuddin, S. S. Herbal drugs Novel drug delivery systems (NDDS). Fitoterapia., 2010; 81, 680-689. doi: 10.1016/j.fitote.2010.05.001
CrossRef - Gaikwad, S. S., & Kshirsagar, S. J. Review on Tablet in Tablet techniques. Beni-Suef University Journal of Basic and Applied Sciences., 2020; 9, 1-7. doi: 10.1186/s43088-019-0027-7
CrossRef - Ma’arif, B., Muslikh, F. A., Amalia, D., Mahardiani, A., Muchlasi, L. A., Riwanti, P., … & Agil, M. Metabolite Profiling of the Environmental-Controlled Growth of Marsilea crenata Presl. and Its In Vitro and In Silico Antineuroinflammatory Properties. Borneo Journal of Pharmacy., 2022; 5(3), 209-228.
CrossRef - Phillipson, D. J. The National Research Council of Canada: Its Historiography, Its Chronology, Its Bibliography. Scientia Canadensis., 1991; 15(2), 177-193. doi: 10.7202/800335ar
CrossRef - Ma’arif, B., Mirza, DM, Suryadinata, A., Muchlisin, MA and Agil, M. Metabolite Profiling of 96% Ethanol Extract from Marsilea crenata Presl. Leaves using UPLC-QToF-MS/MS and Anti-Neuroinflammatory Prediction Activity with Molecular Docking. Journal of Tropical Pharmacy and Chemistry., 2019; 4(6): 261-296. doi: 10.25026/jtpc.v4i6.213
CrossRef - Aditama, APR, Ma’arif, B., Mirza, DM, Laswati, H., Agil, M. In Vitro and in Silico Analysis on the Bone Formation Activity of N-Hexane Fraction of Semanggi (Marsilea crenata Presl.). Sys Rev Pharm., 2020; 11(11): 837-849. doi: 10.31838/srp.2020.11.123
- Sawant, S.S., Gabhe, S.Y., & Singh, K.K. In Vitro Effect on Plasmodium falciparum and In Vivo Effect on Plasmodium berghei of Annomaal, an Oily Fraction Obtained from the Seeds of Annona squamosa. Molecules., 2023; 28 (14), 5472. doi: 10.3390/molecules28145472
CrossRef - Siagian, FE, Isham, D., Ronny, R., Alfarabi, M., Nainggolan, S., Daroedono, E., … & Naibaho, FB. The Profile Of Erythrocyte Enlargement Due To Imported Cases Of Plasmodium Vivax Infection: Its Impact On The Patient And The Community. International Journal of Community Medicine and Public Health., 2020; 7(7). doi: 10.18203/2394-6040.ijcmph20202600
CrossRef - Wijaya SMM, Lisdiana, Setiati, N. Administration of Mango Mistletoe Extract on Codeine-Induced Histological Changes in Rat Liver. Biosciences: Journal of Biology & Biology Education., 2014; 6(2), 80-86. doi: 10.21776/ub.jsmartech.2021.002.02.48
CrossRef - Handani, KS et al. Histopathological Image of Liver of Wistar Rats After Administration of Ethanol Extract of Bangle Rhizome (Zingiber cassumunar Roxb.) in Acute Toxicity Test, Journal of Agromedicine and Medical Sciences., 2018; 4(1), pp. 55–59.
CrossRef - Astuti, NW, Fitrianingsih, SP and Suwendar.Literature Study of the Antimalarial Activity of African Plants (Vernonia amygdalina Del.). Bandung Conference Series: Pharmacy., 2022; 2(2), pp. 1088–1095. doi: 10.29313/bcsp.v2i2.4815
CrossRef - Keawpradub, N., Kirby, G. C., Steele, J. C. P., & Houghton, P. J. Antiplasmodial activity of extracts and alkaloids of three Alstonia species from Thailand. Planta Medica., 1999; 65(08), 690-694. doi: 10.1055/s-1999-14043.
CrossRef - Pandey, K. P., Rahman, M. T., & Cook, J. M. (2021). Bisindole Alkaloids from the Alstonia Species: Recent Isolation, Bioactivity, Biosynthesis, and Synthesis. Molecules (Basel, Switzerland), 26(11), 3459. doi:10.3390/molecules26113459 .
CrossRef - Mustofa, Mustofa & Mallie, Michele & Valentin, Alexis & Lewin, Guy. In vitro Antiplasmodial Activity and Cytotoxicity of Vincadifformine and Its Semisynthetic Derivatives. Indonesian Journal of Biotechnology; Indonesian Journal of Biotechnology-June 2006., 2015; 6. 11. doi:10.22146/ijbiotech.7562.
CrossRef - Addae-Kyereme, J., Croft, S. L., Kendrick, H., & Wright, C. W. Antiplasmodial Activities Of Some Ghanaian Plants Traditionally Used For Fever/Malaria Treatment And Of Some Alkaloids Isolated From Pleiocarpa Mutica; In Vivo Antimalarial Activity Of Pleiocarpine. Journal Of Ethnopharmacology., 2001; 76(1), 99–103. doi:10.1016/s0378-8741(01)00212-4
CrossRef







