Endang L. Widiastuti 1* , Eka Ayu Lailatul Istikomah1
, Eka Ayu Lailatul Istikomah1 , Melisa Intan Barliana2
, Melisa Intan Barliana2 , Nuning Nurcahyani1
, Nuning Nurcahyani1 and Endah Setyaningrum1
and Endah Setyaningrum1
1Department of Biology, Lampung University, Lampung, Indonesia.
2Department of Pharmacy, Padjadjaran University, Bandung, Indonesia.
Corresponding Author E-mail:endang.linirin@fmipa.unila.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2853
Abstract
Many treatments of cervical cancer leave some side effects. Therefore, other cancer treatment which can reduce the side effects must be elaborated, one is by searching of some natural products. Some of the natural products could be obtained from the marine biota such as seagrass, Cymodecea rotundata and Enhalus acoroides. Most of seagrass have bioactive compounds such as alkaloids, flavonoids, saponins and steroids which have antioxidants, antitumor and anticancer activity. The purpose of this study was to determine the activity of the ethanolic extraction of C. rotundata and E. acoroides against HeLa cells. To determine their cytotoxic and antiproliferative activity, we used the CCK-8 assay, with series concentrations of 62.5 ppm, 125 ppm, 250 ppm, 500 ppm, 1000 ppm and 2000 ppm, and doxorubicin as control drug (with incubation times of 24, 48, 72 hours). The results indicated that both extracts had cytotoxic effect on HeLa cell line, with IC50 values of 856.65 ppm (C. rotundata) and 645.96 ppm (E. acoroides). While their anti-proliferative activity indicated by the doubling time of HeLa cell lines, reached 297 hours for C. rotundata at 1000 ppm, and 370 hours for E. acoroids at of 500 ppm. It was concluded that the ethanol extracts of C. rotundata and E. acoroides were cytotoxic and had antiproliferative activity against HeLa cells.
Keywords
Antiproliferative activity; Cytotoxic; Cymodecea rotundata; Enhalus acoroides; HeLa cells
Download this article as:| Copy the following to cite this article: Widiastuti E. L, Istikomah E. A. L, Barliana M. I, Nurcahyani N, Setyaningrum E. Cytotoxic and Antiproliferative Testing of HeLa Cervical Cancer Cells Using Seagrass Ethanolic Extraction (Cymodocea rotundata and Enhalus acoroides ). Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Widiastuti E. L, Istikomah E. A. L, Barliana M. I, Nurcahyani N, Setyaningrum E. Cytotoxic and Antiproliferative Testing of HeLa Cervical Cancer Cells Using Seagrass Ethanolic Extraction (Cymodocea rotundata and Enhalus acoroides ). Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3PfsIfJ |
Introduction
Occurrence of cancer cases tends to increase throughout the years. The highest incidence of cancer cases is in Asia countries, from which in Indonesia itself has a total of 398,914 cases with the highest category is breast cancer at 16.6% and followed by cervical cancer at 9.2%1. From Ministry of Health of Indonesian Republic, Cervical cancer is a type of cancer that develops in the cervix which is in the lower third of the uterus and is connected to the vagina through the external uterine ostium2. This happens because of the presence of the HPV virus (Human Papilloma Virus). HPV 16 and 18 are oncogenes which are responsible for 70-80% of cervical cancer cases in the world3.
Currently there are several ways of treating cervical cancer such as removal of localized cancer tissue (surgery), irradiation of radiation, and chemotherapy. Each of these treatments is carried out according to the type and stage of cancer when diagnosed4. However, this therapy has side effects such as scarring after surgery, experiencing alopecia, nausea, emesis, anemia, fatigue, infection, infertility, menopause, weight changes, hepatotoxicity and triggers cancer in other organs5. Therefore, searching other alternative treatments must be conducted, one of which is by utilizing natural ingredients such as seagrass Cymodecea rotundata and Enhalus acoroides which have to be known contain some bioactive compounds such as alkaloids, flavonoids, saponins and steroids6 as drugs, antioxidants, antitumor and anticancer7. Previous study8 indicated stated that methanolic extraction of Enhalus acoroides had potential as an anticancer by performing cytotoxic anti-proliferative activity on HeLa cell line. Based on that previous study, the aim of this study was to further analyze the action of cytotoxic and antiproliferative activity of other sea grasses, namely Cymodecea rotundata as well as Enhalus acoroides against HeLa cell line. But at this study ethanol was used to extract some bioactive from two different sea grasses, since ethanol is the most commonly compound used for bioactive extraction of many plants.
Material and methods
This research was conducted from September to June 2023. Cymodecea rotundata was obtained from the Tegal Perak beach, Pesawaran Regency of Lampung Indonesia and Enhalus acoroides was obtained from Dollar Beach Padada beach, South Lampung Regency of Lampung Indonesia. The seagrass obtained was cleaned with running water, dried, extracted by maceration method using 96% ethanol with a ratio of 1:10 for 24 hours. The obtaining extract then were evaporated using a rotary evaporatory with a temperature of 50°C until a thick extract was obtained9. Then the phytochemical determination was carried out with the following procedure:
Table 1: Procedure for Determining Secondary Metabolites10
|
Test type |
Treatment |
Indicator |
|
Alkaloids |
0.5 ml sample was used then 5 drops of chloroform was added followed by 5 drops of Mayer’s reagent (1 g of KI dissolved in 20 ml dH2O and added by 0.271 g HgCl2) |
The color of the solution is brownish white |
|
Flavonoids |
0.5 ml sample was added with 0.5 g Mg and 5 ml concentrated HCl (added drop by drop) |
The color of the solution is red or yellow in the form of foam |
|
Saponins |
0.5 ml of sample was added with 5 ml of dH2O, shaken well for 30 seconds |
Formed foam |
|
Steroids |
0.5 ml sample was added with 0.5 ml glacial CH3COOH and 0.5 ml H2SO4 |
The color of the sample changes to blue or purple |
|
tannins |
1 ml of sample was added with 3 drops of FeCl3 solution |
The color of the solution becomes black |
HeLa cell culture media was prepared according to procedure of CCRC12. At room temperature, 5 ml of 10% Fetal Bovine Serum (FBS) and 0.5 ml Penicillin Streptomycin (Pensterp) was added and blended in a sterilized bottle (to thaw cell line), then followed by adding DMEM (Dulbecco’s Modified Eagle’s Medium) up to 50 ml.
HeLa cell cultures were taken from liquid nitrogen tanks and then thawed in a water bath at 37°C for 3 minutes. By using a sterile conical tube containing 10 ml of DMEM culture medium, cells were placed and incubated for 4 hours at 36°C. The cancer cells then were centrifuged for 5 minutes at 1500 rpm to separate from the medium. The obtaining supernatant was removed and HeLa cells were grown in 4 tissue culture flasks containing 10% FBS dissolved in DMEM. The flasks were kept at 36°C with 5% CO2 flow and lid flask loosened to optimize aeration for cell growth. The media was replaced after 3 days and the cells were grown again until 80% concentration was sufficiently for treatments11.
HeLa cell harvesting was carried out after the cells reached 80% confluence as indicated by the cells filling the tissue culture flask, then the cells were released from the flask wall by aspirating the media using a sterile Pasteur pipette. Five (5) ml of PBS was used to wash cultured cells and it was repeated twice. Cells then were added with 0.25% trypsin EDTA solution to release cells and again they were incubated in a CO2 incubator at 36°C for 5 minutes. The cells then were added by 5 ml of media which had been mixed with 10% FBS and Pensterp, and then re-suspended again with a pipette until it did not show any clotting. The re-suspended cells were then put into a sterile conical tube11.
Cell count. The cells in the conical tube were then filled with 3 ml of media. Then the cells were centrifuged for 5 minutes at 1500 rpm. The supernatant was discarded and 10 ml of fresh media was added to the precipitated formed nathan. Cells as much 10 μl was pipetted into the well plate and was added by 10 μl of trypan blue. The number of cells was calculated, 10 μl of the mixed cells was pipetted into a hemocytometer. Live cells were colorless or clear, while non-living cells were blue. Cell count with a hemocytometer was carried out by selecting 4 counting chambers under a microscope. A series of calculations for determining the number of cells to be cultured was followed12,13.

Number of cells counted/ml = mean cells x dilution factor x 10 4
Total number required cells = number of wells x number of cells per well
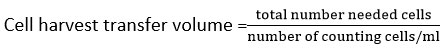
The cell harvest volume calculations then were transferred into the conical tube and media was added.
Stock solution preparation
The ethanol extracts of Cymodocea rotundata and Enhalus acoroides were performed prior to the cytotoxicity and antiproliferation tests. The stock solution was prepared by dissolving 10 mg of the extract with 1 ml of 5% dimethyl sulfoxide (DMSO). Then, it was put in a bath at 30°C until the extract dissolves. The stock solution was put into closed sterile microtubes and stored in the refrigerator prior used. For the study treatments, the stock solution then was diluted again to concentrations of 2000 ppm, 1000 ppm, 5000 ppm, 250 ppm, 125 ppm and 62.5 ppm, either for Cymodocea rotundata and Enhalus acoroides. As for Doxorubicin (used as control treatment), we used concentrations of 20 ppm, 10 ppm, 5 ppm, 2.5 ppm, 1.25 ppm, and 0.625 ppm. The prepared Doxorubicin and extract solutions in various concentrations were then tested on HeLa cells in a Laminar Air Flow Cabinet12.
Cytotoxic activity
For the cytotoxic evaluation, the final harvested volume of cells was used and affixed to the medium. They were loaded into each well plate as much as 100 μl followed by incubation in 5% CO2 incubator for 24 hours at 36°C aiming that the cells would stick to the wall of the flask12. The 24 hour- cultured cells were removed from the incubator and then perceived under a microscope. Prior rinsing with PBS solution, the culture medium was discarded. Each well plates then was given sea grass extract of 50 µl of each treatment concentrations (62.5 ppm, 125 ppm, 250 ppm, 500 ppm, 1000 ppm and 2000 ppm for both sea grasses extract) as well as for Doxorubicin at concentration of 0.625 ppm, 1.25 ppm, 2.5 ppm, 5 ppm, 10 ppm and 20 ppm. Meanwhile for the control cells, they were given DMSO which used as solvent. They were then incubated again in a 5% CO2 incubator at 36°C for 24 hours. After incubation time, medium was removed from each well plates and each well plates was rinsed using PBS and given 50 µl of CCK-8 reagent and reared again in a CO2 incubator at 36°C for 2 hours. After incubation for 2 hours, viability of the cells of each well plates were determined by using ELISA reader at a wavelength of 450 nm13,14. To obtain HeLa cell viability (in percent), the following formula was used13.

The percentage value of cell viability was then converted into a probit value to determine the IC50 value using the Microsoft Excel program.
Antiproliferation activity determination
A 100 μl of cell suspension was filled to each well plates followed by incubation in a CO2 incubator 24 hours at 37°C. After 24 hours of incubation, the cells were removed from the incubator and then perceived under a microscope. After discarding culture media, cells were washed with PBS solution. With the same procedure as those of cytotoxic activity determination, each well plates were incubated a 5% CO2 incubator at 36°C within different time treatments, namely 24, 48 and 72 hours. After the incubation time was attained, the test solution was removed, and the well plates were washed again with PBS solution and given with 50 μl of CCK-8 reagent and again incubated in a CO2 incubator at 36°C for 2 hours. After incubation for 2 hours, viability of the cells of each well plates were determined by using ELISA reader at a wavelength of 450 nm13,14,15. In the anti-proliferation test, data processing was carried out to determine the difference in the number of living cells from extract treatment with numerous concentrations. Meanwhile the different incubation times was used to determine the doubling time value for each treatment using the following formula16:
Double time =
In which Y = Log (2 x Number of initial living cells); A = Intercept; B = Slopes
Results and Discussion
The weight of simplisia and the pasta of the sea grasses, Cymodocea rotundata and Enhalus acoroides, extraction could be seen in Table 2. This result was needed to show how much percentage that we could gain from 100 gram of simplisia (dry mass of both sea grasses) which seemingly that both had similar percentage of less than 1 %.
Table 2: The mass extract of Cymodocea rotundata and Enhalus acoroides
|
No |
Seagrass |
Sample Mass (grams) |
Percent (%) |
|
|
Simplisia |
Pasta |
|||
|
1 |
Cymodocea rotundata |
100 |
4.586 |
0.046 |
|
2 |
Enhalus acoroides |
100 |
4.952 |
0.049 |
While the phytochemical tests (qualitatively, just to indicate that some bioactive was visible) on the extracts of Cymodocea rotundata and Enhalus acoroides waspresented in table 3 as follows:
Table 3: Phytochemical content of Cymodocea rotundata and Enhalus acoroides extracts
|
No. |
Phytochemical Test |
Cymodocea rotundata |
Enhalus acoroides |
|
1 |
Alkaloids |
+ |
+ |
|
2 |
Flavonoids |
+ |
+ |
|
3 |
Saponins |
+ |
+ |
|
4 |
Steroids |
+ |
+ |
|
5 |
tannins |
+ |
– |
Note: +/-: Indicating of such bioactive compound exist or not
The cytotoxic activity of the ethanolic extraction of sea grasses, Cymodocea rotundata and Enhalus rotundata by using CCK-8 assay could be seen in Fig 1 and 2 as follows.
 |
Figure 1: Cytotoxic of Cymodocea rotundata ethanol extract on HeLa Cell line |
Percentage of HeLa cell viability was reduced in treatment using the ethanol extract of Cymodocea rotundata compared to control cells. Meanwhile, Cymodocea rotundata at concentrations of 1000 ppm and 2000 ppm, was able to sharply reduce the viability of HeLa cells, even compared to the Doxorubicin at the highest concentration in this study (5 – 10 ppm). The decrease in cell viability at these concentrations was 97.61% and 99.02%. This indicated that ethanolic extraction of Cymodocea rotundata contained of bioactive which presumably could be elaborated to be one of potential anticancer drug from one of marine plants. Yet, further much deeper study is needed.
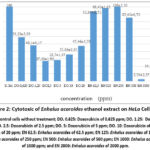 |
Figure 2: Cytotoxic of Enhalus acoroides ethanol extract on HeLa Cell line |
As well as those seen in Cymodocea rutundata, the ability of Enhalus acoroides ethanolic extract to suppress cell viability at concentrations of 1000 ppm and 2000 ppm was higher than those of the control group and Doxorubicin treated groups. The percentage of decreasing in cell viability at these concentrations was 97.44% and 85.48%. Again, this indicated that ethanol extract of Enhalus acoroides at these two concentrationa were more toxic than those of control drug using Doxorubicin.
In order to determine the IC50 value of both sea-grasses ethanolic extract, linear regression was plotted for both sea-grasses ethanolic extraction, which could be seen in Figures 3 and 4 as follows. From these figures (Fig. 3, 4), it could be seen that the extracts of Cymodocea rotundata and Enhalus acoroides were both able to decrease cell viability of HeLa with each IC50 values less than 1000 ppm. Beside, the IC50 value of the Enhalus acoroides extract was also lower than that of Cymodocea rotundata extract.
Cytotoxic activity of HeLa cells using Cymodocea rotundata and Enhalus acoroides extracts were carried out to test the toxic potential of the extracts used using the test parameter, namely IC50 (50% inhibitory concentration)17. IC50 is the concentration used to inhibit or inhibit the activity of cancer cells. The smaller the IC50 value obtained, the better some compound to have alternative potential as an anticancer drug by inhibiting as much as 50% of the proliferative activity of the cancer cells themselves14. It is known that the ethanolic extract of Enhalus acoroides had an IC50 value of 645.96 ppm, which was lower than Cymodocea rotundata of 856.65 ppm.
According to the NCI (National Cancer Institute) it is known that there are categories of compounds that are considered to be toxic. The categories are seen from their IC50 values based on U.S. National Cancer Institute (NCI) and Geran protocol, which are as follows: IC50 ≤ 20 μg/ml = high, IC50 21-200 μg/ml = moderate, IC50 201-500 μg/ml = weak and IC50 > 501 μg/ml = no toxic18. Based on these categories, the ethanol extracts of Cymodocea rotundata and Enhalus acoroides of this study was included in compounds that was not toxic but both does have the potential as anticancer agents to inhibit HeLa cell growth. This is in accordance with the finding of other study19 which states that if a compound had an IC50 value of <1000 μg/ml then the compound has the potential as an anticancer.
Differences in IC50 cytotoxic values in sea-grasses extract of Cymodocea rotundata and Enhalus acoroides could be accounted from the differences in bioactivity produced from both sea-grasses, from which can be also influenced by their environment (chemical and physical condition) where they grow. These differences were thought to affect differences in chemical content so that the bioactivity could also be different20. Sea-grasses Cymodocea rotundata has a special feature where the leaf edges are not serrated, and the leaf sheaths are closed. The rhizomes of this sea-grass are smooth, and have irregularly branched roots. This sea-grass habitat is on a muddy sand substrate. Whereas the Enhalus acoroides sea-grass has a feature where its largest size can reach 1 meter and there are hairs on its rhizomes. Enhalus acoroides usually grows and lives in tidal areas21.
Besides being influenced by their environment, the cytotoxic ability of Cymodocea rotundata and Enhalus acoroides is also influenced by the secondary metabolites contained therein. Cymodocea rotundata and Enhalus acoroides are known to contain active compounds such as alkaloids, flavonoids, saponins and steroids. In addition, Cymodocea rotundata is also known to contain tannins as those also indicated from its phytochemical tests. Tannins contained in Cymodocea rotundata are thought to have anticancer activity by inhibiting tyrosine kinase and also as antioxidants. Tannins also have mechanisms that can inhibit enzymes such as transcriptase and DNA topoisomerase22. Other study also indicated that Cymodocae rotundata contained coumarins, flavonoids, phenols, proteins, free amino acids, quinones, saponins, sterols, sugars, terpenoid bioactive compounds with antibacterial, cytotoxic and hemolytic activity23.
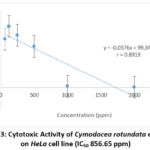 |
Figure 3: Cytotoxic Activity of Cymodocea rotundata extract |
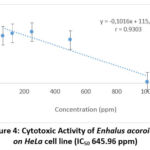 |
Figure 4: Cytotoxic Activity of Enhalus acoroides on HeLa cell line (IC50 645.96 ppm). |
The antiproliferation activity of both sea-grasses could be seen in Figure 5 (a, b) as follows:
 |
Figure 5: Cell viability at 24, 48, and 72 hours of incubation of ethanol extract (a) Cymodocea rotundata (b) Enhalus acoroides |
The highest average number of living cells in Cymodocea rotundata extract was found at 62.5 ppm group at 24 hours of incubation. The lowest number of live cells was found at a concentration of 500 ppm at 48 hours. This showed that given of Cymodocea rotundata had a significant effect on HeLa cell death because the higher the concentration and time, the lower the living cell average. In the treatment of Enhalus acoroides extract there was a significant difference among treatment groups with the highest number of living cells was obtained from 2000 ppm at 24 hours of incubation. The lowest cell count was at a concentration of 500 ppm and within 24 hours.
Furthermore, the calculation of the doubling time value was carried out to determine each treatments on the proliferative ability of HeLa cells. The results of the doubling time values obtained after being treated with the ethanolic extracts of Cymodocea rotundata and Enhalus acoroides could be seen in Table 4 as follows:
Table 4: Doubling time of HeLa Cell line given extracts of C. rotundata andE. acoroides
|
Sea-grass |
Concentration (ppm) |
Incubation Time Equations with Logs of Cell Count |
Slope |
Value of doubling time (hours) |
|
Cymodocea rotundata |
2000 |
Y= 6973.2 + -18741 |
6973,2 |
269 |
|
1000 |
Y= 11232 + -33386 |
11232 |
297 |
|
|
500 |
Y = 2499 + -522.5 |
2499 |
21 |
|
|
250 |
Y= 24515 + -83222 |
24515 |
339 |
|
|
125 |
Y= 50867 + -197726 |
50867 |
389 |
|
|
62.5 |
Y= 65162 + -261294 |
65162 |
401 |
|
|
Enhalus acoroides |
2000 |
Y= 44314 + -167948 |
44314 |
379 |
|
1000 |
Y= 7039.7 + -21094 |
7039.7 |
300 |
|
|
500 |
0 |
0 |
0 |
|
|
250 |
Y= 33696 + -124783 |
33696 |
370 |
|
|
125 |
Y= 46270 + -177917 |
46270 |
385 |
|
|
62.5 |
Y= 57926 + -229609 |
57926 |
396 |
|
|
Cell Control |
0 |
Y= 42842x – 16422 |
42842 |
38 |
It was seen that the doubling times obtained in the treatment of Cymodocea rotundata and Enhalus acoroides ethanolic extracts were different. The slope value of Cymodocea rotundata ethanol extract at concentrations of 62.5 ppm and 125 ppm were greater than the control cells with doubling time values of 401 hours and 389 hours. In the ethanol extract of Enhalus acoroides it was found that the slope values at concentrations of 62.5 ppm, 125 ppm, and 2000 ppm had a greater slope value than the control group with doubling time values of 396 hours, 385 hours, and 379 hours. The smaller the slope value obtained the longer the time required for doubling the time and as well as the greater the slope value of the treatment groups compared to the control cell, the shorter the doubling time is.
The antiproliferative ability of the ethanol extracts of Cymodocea rotundata and Enhalus acoroides showed in longer doubling time than control cells. This means that the ethanol extracts of Cymodocea rotundata and Enhalus acoroides were able to inhibit the growth speed at which HeLa cervical cancer cells multiply. If such compound could delay the doubling time of cells, then there was possibility that the compound could inhibit the genes and proteins which regulate the cell cycle. Presumably, the inhibition of cell proliferation was caused by the mechanism of secondary metabolites contained in the ethanol extract of Cymodocea rotundata, Enhalus acoroides such as alkaloids, flavonoids, saponins, steroids and tannins. Alkaloids, which also found in seagrass Cymodocea rotundata and Enhalus acoroides, are known to have anticancer activity24. From which alkaloids could act as antiangiogenic, antiproliferative, inhibit topoisomerase activity, tubulin polymerization and induce apoptosis24. Alkaloids also can increase apoptosis by inducing DNA damage25. Alkaloids also have potential as antioxidants by donating H atoms to free radicals, so that free radicals will be stable26.
Flavonoid compounds are usually found in several plants and are used as disease prevention, one of which is as an anticancer by inhibiting cell proliferation through inhibiting oxidative processes which can initiate cancer cells in the body. Presumably the enzymes xanthin oxidase, Cyclooxygenase (COX) and Lipooxygenase (LOX) was dropped, causing slowing down in cell cycle27. Flavonoids affect the induction of apoptosis by increasing the activity of caspase 3 and cox 228. In addition, the enzyme expression for topoisomerase I and II was also inhibited. The topoisomerase complex will be alleviated then by topoisomerase enzyme inhibitor triggering DNA to be cut and damaged29. Tannins have activity as free radical scavengers and are able to inhibit lipoxygenase and lipid peroxidase. Tannins inhibit the S phase or cell cycle synthesis. The cell will carry out DNA synthesis and chromosome replication in the S phase30. Based on the compounds contained in the seagrass Cymodocea rotundata and Enhalus acoroides, the two seagrasses can be used as anticancer agents based on the IC50 value for inducing apoptosis and the doubling time value on the proliferative ability of HeLa, cervical cancer cells.
Conclusion
From this study we can conclude that the activity of the ethanolic extraction of Cymodocea rotundata and Enhalus acoroides was cytotoxic against HeLa cervical cancer cells, as evidenced by reducing viability cell percentage related to the control group with IC50 values of 856.65 ppm and 645.96 ppm. The ethanol extracts of Cymodocea rotundata and Enhalus acoroides also have antiproliferative against HeLa cells. This can be seen in the longer doubling time compared to the control cells. Based on this, the ethanol extracts of Cymodocea rotundata and Enhalus acoroides have potential as HeLa cervical anticancer agents.
Acknowledgement
We deeply appreciated to the Research and Community Service Institute (LPPM) of University of Lampung and Graduate University of Lampung and to the CC&C Laboratory-Faculty of Pharmacy, UNPAD (Padjajaran University-Indonesia) for helping us carry out this research.
Conflict of Interest
There is no conflict of interest on publishing this article.
Funding Source
The source of funding this research was supported by the University of Lampung under the Research and Community Service Institute (LPPM – UNILA) through BLU DIPA with contract number 861/UN26.21/PN/2023.
References
- GLOBOCAN. Indonesia – Global Cancer Observatory. 2020. https://gco.iarc.fr/
- Kemenkes RI. Profil Kesehatan Indonesia. Kemenkes RI. 2017. Jakarta.
- Jaspers L, Budiningsih S, Wolterbeek R, Henderson F, Peters A. Parental acceptance of human papillomavirus (HPV) vaccination in Indonesia: A cross-sectional study. Vaccine. 2011; 29:7785-7793.
CrossRef - Pusdatin Kemenkes RI. Situasi penyakit Kanker. Kemenkes RI. 2015. Jakarta.
- Satyarsa, A B.S. Studi Pustaka: Potensi Fucoidan dari Rumput Laut Coklat (Sargassum sp.) sebagai Inovasi Terapi pada Kanker Payudara. Journal of Medicine and Health. 2019;2(3): 909-919
- Nurafni, N R M. Identifikasi Senyawa Bioaktif Jenis-Jenis Lamun Di Perairan Pulau Morotai. Seminar Nasional Pendidikan Biologi Kepulauan Aula Banau, Ternate. E-ISSN 2623-2146. 2018; 26-32.
- Robinson, T. Kandungan Organik Tumbuhan Tinggi. ITB. Bandung. 1995.
- Widiastuti, E L., Rima, K., Busman, H. Anticancer Potency of Seagrass (Enhalus acoroides) Methanol Extract in The HeLa Cervical Cancer Cell Culture. Proceedings of the International Conference on Sustainable Biomass. 2019; 202:38-42.
- Nurfitri, W. A., Widiastuti, E. L., & Cahyani, E. N. Efek Ekstrak Metanol Daun Jeruju (Acanthus ilicifolius L.) Serta Buah Jeruju Dan Taurin Dalam Menurunkan Kadar Glukosa Darah Dan Kolesterol Serta Fertilitas Mencit Jantan (Mus musculus) Yang Diinduksi Aloksan. Prosiding Seminar Nasional Tumbuhan Obat Indonesia Ke-55. 2018:267–275.
- Tasmin, N. Isolasi, Identifikasi Dan Uji Toksisitas Senyawa Flavonoid Fraksi Kloroform Dari Daun Terap (Artocarpus Odoratissimus Blanco). Jurnal Kimia Mulawarman. 2014; 12(1).
- Junedi S. Prosedur Tetap Panen Sel, Cancer Chemoprevention Research Center Fakultas Farmasi UGM. 2009:1-3.
- CCRC. Prosedur Uji Proliferasi Sel (Doubling time). Fakultas Farmasi Universitas Gajah Mada. 2009. Yogyakarta.
- CCRC. Prosedur Uji Sitotoksik dengan Metode MTT (3-4,5-dimetiltiazol-2- il)-2-5-difenil tetrazoliumbromida). Universitas Gajah Mada. 2013. Yogyakarta.
- Dona, R., Frimayanti, N., Ikhtiarudin, I., Iskandar, B., Maulana, F., Silalahi, N T. Studi In Silico, Sintesis, dan Uji Sitotoksik Senyawa P-Metoksi Kalkon Terhadap Sel Kanker Payudara MCF-7. Jurnal Sains Farmasi dan Klinis. 2019; 6(3): 243-249.
CrossRef - Ace Biolabs. CCK-8 Cell Counting Kit. Ace Biolabs CO., LTD. Taiwan.
- Nuraini, L H. 2011. Uji Sitotoksisitas Dan Antiproliferatif Fraksi Etil Asetat Ekstrak Etanol Biji Jinten Hitam (Nigella sativa, Lour) Terhadap Sel Mieloma. Jurnal Ilmiah Kefarmasian. Vol 1 No 2: 11-21.
CrossRef - Rahardhian, M R R., Utami, D. Uji Sitotoksik Dan Antiproliferasi Ekstrak Eter Daun Binahong (Andredera cordifolia (Tenore) Steen.) Terhadap Sel HeLa. Media Farmasi Indonesia. 2018; 13(1):1284-1292.
- Niksic H, Becic F, Koric E, Gusic I, Omeragic E, Muratovic S, Miladinovic B, Duric K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci Rep. 2021;11(1):13178. doi: 10.1038/s41598-021-92679-x. PMID: 34162964; PMCID: PMC8222331.
CrossRef - Lisdawati, V. Senyawa Lignan dari Fraksi Etil Acetat Daging Buah Mahkota Dewa Phaleria macrocarpa (Scheff.). Boerl. Thesis. 2002. Universitas Indonesia. Jakarta.
- Nur, S., Baitanu, J. A., & Gani, S. A. Pengaruh Tempat Tumbuh dan Lama Penyulingan secara Hidrodestilasi terhadap Rendemen dan Profil Kandungan Kimia Minyak Atsiri Daun Kemangi (Ocimum canum Sims L.). Jurnal Fitofarmaka Indonesia. 2019; 6(2):363-367.
CrossRef - Pranata, A., I. N. Suwastika., P. Paserang. Jenis – jenis lamun ( seagrass ) di kecamatan tinangkung, banggai kepulauan , sulawesi tengah. Natural Science: Journal of Science and Technology. 2018;7(3): 349–357.
- Salim. Penentuan Daya Inhibisi Ekstrak air dan Etanol Daging Buah Mahkota Dewa (Phaleria macrocarpa (Scheff.) Boerl.) Terhadap Aktifitas Enzim Tirosin Kinase Secara In Vitro. Skripsi. Bogor: Departemen Kimia, Fakultas MIPA, IPB. 2006.
- Ragupathi Raja Kannan R, Arumugam R, Iyapparaj P, Thangaradjou T, Anantharaman P. In vitro antibacterial, cytotoxicity and haemolytic activities and phytochemical analysis of seagrass from the Gulf of Mannar, South India. Food Chem. 2013;136: 1484-1489.
CrossRef - Tohme, Y., Sakaki, K., Shinozaki. M. Isthani. Genetics From Genes to Genomes. Edisi ke 4. New York: The mcGraw Hill. 2011: p 154.
- Habli, Z., Toumieh, G., Fatfat, M., Rahal, O. N., & Gali-Muhtasib, H. 2017. Emerging Cytotoxic Alkaloids In The Battle Against Cancer: Overview Of Molecular Mechanisms. Molecules, 22(2), 250.
CrossRef - Melinda, S., Annisaa’, E., Sasikirana, W. Potensi Sitotoksik Ekstrak Buah Parijoto (Medinilla speciosa) Terpurifikasi pada Sel Kanker Serviks HeLa. Journal of Research in Pharmacy. 2021; 2(1): 47-55
CrossRef - Ismaryani, A., Salni., Setiawan, A., Triwani. 2018. Aktivitas Sitotoksik, Antiproliferasi dan Penginduksi Apoptosis Daun Salung (Psychotria viridiflora Reinw. Ex. Blume) terhadap Sel Kanker Serviks HeLa. Jurnal Ilmu Kefarmasian Indonesia. Vol 16 No 2: 206-213.
CrossRef - Ulfa, A.M., Herdwiani1, W., Purwidyaningrum, I. Kajian Literatur Aktivitas Antikanker Tanaman Bunga Matahari (Helianthus Annus L) Terhadap Berbagai Kultur Sel Kanker. Pharmacy Medical Journal. 2021; 4(2):73-82.
CrossRef - Purnamasari, M. Efek Ekstrak Metanol Daun Kersen (Muntingia calabura L.) Terhadap Induksi Apoptosis Sel Kanker WiDr. Proceedings of Continuing Medical Education, Workshop and Symposium Maternity: Medical Update Emergency Obstetry and Gynecology in the Primary Care, Univ. Muhammadiyah-Surakarta. 2019.
- Ahmed, N., Vasantha, K. S., John, A. K., Shobana, C., & Usharani, B. Anticancer activity of hydroalcoholic extract of Enhalus acoroides. International Journal of Health Sciences. 2022; 6(S1):9528–9537.
CrossRef








