Neny Purwitasari1,2 and Mangestuti Agil1*
and Mangestuti Agil1*
1Department of Pharmaceutical Sciences, Faculty of Pharmacy, Universitas Airlangga, Surabaya-Indonesia
2Doctoral Program, Faculty of Pharmacy, Universitas Airlangga, Surabaya
Corresponding Author E-mail: mangestuti@ff.unair.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2540
Abstract
Bidara upas (Merremia mammosa (Lour.) Hallier f.) tuber is empirically used to treat respiratory disorders and tuberculosis. The pharmacological effect is due to the activity of various secondary metabolites. This study aims to determine the metabolite profile of M. mammosa tuber ethanol extract, n-hexane fraction, ethyl acetate fraction, and n-butanol fraction. The dried powder of the tuber of M. mammosa was extracted with 96% ethanol. Then, liquid-liquid fractionation was performed using n-hexane, ethyl acetate, and butanol as solvents. As much as 5 µl of each sample was injected into the UPLC-QToF-MS/MS and analyzed using the MassLynx 4.1 software and the ChemSpider and MassBank online databases. After identifying each compound, information regarding its activity was retrieved from the scientific literature. Metabolite profiling revealed that the 96% ethanol extract of M. mammosa yielded 61 compounds, with the n-hexane fraction yielding 64 compounds, the ethyl acetate fraction yielding 54 compounds, and the butanol fraction yielding 44 compounds. According to the findings of this study, the metabolite profiles of each M. mammosa tuber extract and fractions were distinct. Several compounds in these extracts and fractions may have antiviral, antioxidant, anti-inflammatory, and other properties; hence, more studies are required to determine their potential.
Keywords
Metabolite profiling; Merremia mammosa (Lour.) Hallier f; tuber; UPLC-QToF-MS/MS
Download this article as:| Copy the following to cite this article: Purwitasari N, Agil M. Metabolite Profiling of Extract and Fractions of Bidara Upas (Merremia Mammosa (Lour.) Hallier F.) Tuber Using UPLC-QToF-MS/MS. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Purwitasari N, Agil M. Metabolite Profiling of Extract and Fractions of Bidara Upas (Merremia Mammosa (Lour.) Hallier F.) Tuber Using UPLC-QToF-MS/MS. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3F6AfHP |
Introduction
Acute Respiratory Syndrome, Severe Coronavirus 2 (SARS-CoV2), causes COVID-19, a severe acute respiratory disease1. In December 2019, the COVID-19 pandemic was first detected in Wuhan, Hubei Province, China. As of 2 August 2022, the World Health Organization (WHO) has recorded 575,887,049 confirmed COVID-19 cases, including 6,398,412 deaths2. The SARS-CoV-2 virus attacks humans via breathing and damages ciliary cells in the lungs. Coronavirus infection causes fever, dry cough, vertigo, nausea, shortness of breath, and diarrhea3.
According to the World Health Organization (WHO), there are no viable antiviral for SARS-CoV2. Diverse efforts, including social distancing, testing, and tracking, as well as research and development of medicines and vaccines, are employed to prevent the fast spread of this coronavirus 4. The basic treatment for SARS-CoV2 is supportive care supplemented with broad-spectrum antibiotics, antivirals, corticosteroids, and convalescent plasma. The recent medication, Avigan (favipiravir) and Chloroquine, have severe adverse effects and have not yet been approved as a primary treatment5,6. To combat SARS-CoV2, a chemical-based, natural, effective, and safe alternative medicine is required.
Our ancestors have used herbal medicine derived from medicinal plants for centuries against different diseases and pandemics. As mentioned in several ancient manuscripts, textbooks, and pharmacopeias, medicinal plants or herbs have effectively treated and cured various diseases for hundreds or even thousands of years. Herbal medications are considered one of the alternative COVID-19 treatments7-9. The contribution of Traditional Chinese Medicine (TCM) to the recovery of COVID-19 patients inspires the optimism that Indonesian medicinal plants can also prevent COVID-19 infection and promote the healing of COVID-19 patients10,11.
M. mammosa belongs to the Convolvulaceae family and is synonymous with Convolvulus mammosus Lour. This plant can reach 3-6 m and grow to creep or twist. The tubers resemble sweet potatoes and are edible. Place the harvested tubers in the ground. The tuber has yellow-brown, thick, and gelatinous skin. When dry, the tubers become brown12. M. mammosa tuber is used empirically to treat respiratory illnesses and tuberculosis12,13. The anti-tuberculosis efficacy has been proven in vitro, showing that M. mammosa tuber extract can serve as an inhibitor of Mycobacterium tuberculosis and has no toxicity to experimental animals13. According to its phytochemical study, this plant possesses glycoside resins, tropane alkaloids, polyphenols, and flavonoids 14. Researchers discovered that glycoside resins and flavonoids block cyclooxygenase and lipoxygenase cycles in the inflammatory process15,16. In addition, the flavonoid content of M. mammosa tubers has an antioxidant effect by boosting the activity of Superoxide Dismutase (SOD) and glutathione transferase, thereby decreasing the number of reactive oxygen species (ROS) that can cause cell damage. In addition, alkaloids, flavonoids, and tannins have antibacterial properties by inhibiting bacterial cell proliferation17.
Metabolite profiling, one of the metabolomics-based analyses that describes the profile of secondary metabolites in plants, was performed to determine the metabolite components in the M. mammosa tuber18. UPLC-QToF-MS/MS was utilized to characterize the metabolite profiles in this study. UPLC-QToF-MS/MS is a chemical analysis method that integrates the physical separation capabilities of liquid chromatography and mass spectrometry. This apparatus has various advantages, such as increasing the efficiency of compound separation; lowering the analysis time; requiring fewer samples; offering more accurate monoisotopic mass measurements, having high-resolution spectra for compound confirmation; and producing results19,20. Using UPLC-QToF-MS/MS to study the metabolite profiles of 96% ethanol extract, n-hexane fraction, ethyl acetate fraction, and butanol fraction from M. mammosa tuber can serve as a database for future studies.
Materials and Methods
Plant material
The M. mammosa tuber was collected in Sumenep, Indonesia. The plants were subsequently identified and characterized at UPT Materia Medika, Batu, Indonesia with specimen No 074/674/102.20-A/2022. The tuber was cut into slices, dried, and ground into powder.
Chemicals substances
The 96% ethanol, n-hexane, ethyl acetate, butanol, acetonitrile, and formic acid used as solvents, and mobile phases in UPLC-QToF-MS/MS were purchased from Merck (Darmstadt, Germany).
Extraction
The powder of the M. mammosa tuber was extracted for 3 x 2 minutes using 96% ethanol and an ultrasonic process (Soltec Sonica 5300EP S3, Italy). The filtrate was then evaporated at 50 °C and 70 revolutions per minute using a Heidolph G3 rotary evaporator.
Fractionation
The 96% ethanol M. mammosa tuber extract was suspended in water at a 1:10 ratio. The water suspension (aqueous phase) was then mixed with n-hexane at a 1:1 ratio on a separating funnel for liquid-liquid fractionation. Multiple times of shaking were performed. The n-hexane fraction of M. mammosa tuber was obtained by rotational evaporation of the n-hexane phase, which had been separated from the aqueous phase. The aqueous phase, extracted and separated from the n-hexane phase, is mixed with ethyl acetate at a 1:1 ratio on a separating funnel for liquid-liquid fractionation using the same techniques. In addition, the same techniques were utilized to extract the butanol fraction from the M. mammosa tuber.
Metabolite profiling
Metabolites profile utilizing the UPLC-QToF-MS/MS equipment and metabolite profiling were conducted at the Forensic Laboratory Center of the Indonesian National Police Criminal Investigation Agency. Extracts and fractions were obtained using the Solid Phase Extraction (SPE) method. As much as 5 µl of each sample was then injected into an MS Xevo G2-S QToF detector-equipped ACQUITY UPLC® H-Class System (Waters, USA) (Waters, USA). At a flow rate of 0.2 ml/min, the samples were separated on an ACQUITY BEH C18 column (1.7 m; 2.1 50 mm) using acetonitrile + 0.1% formic acid and water + 0.1% formic acid as the mobile phase. Using the MassLynx 4.1 software, chromatogram data and m/z spectra were retrieved from the UPLC-QToF-MS/MS analysis results for each observed peak. ChemSpider and MassBank were utilized to validate the newly found chemicals.
Results and Discussion
The chemical content of the M. mammosa tuber extract and fractions was predicted using metabolite profiling48. Utilizing the UPLC-QToF-MS/MS instrument, metabolite profiling was performed. Previously, M. mammosa tuber extract and fractions were produced using Solid Phase Extraction (SPE). This technique is the most common sample preparation method for studying novel pharmaceutical chemicals and metabolites in biological matrices. It is utilized to purify a sample before employing chromatographic or another analytical technique to quantify the number of analytes in the sample49. SPE is quite helpful because it removes contaminants from the sample, increasing the spectral sensitivity50,51.
In order to avoid introducing bias into the sample identification, the total ion chromatogram (TIC) of the compound of the sample was first determined. Each TIC peak corresponds to the presence of a single chemical. The mass spectrum of each TIC peak was examined using MassLynx 4.1 and confirmed using the internet databases ChemSpider and MassBank.
Figures 1, 2, 3, and 4 display the findings of UPLC-QToF MS/MS metabolite profiling of M. mammosa tuber extracts and fractions as TIC. Tables 1, 2, 3, and 4 present the retention time (RT), percent area, m/z, chemical formula, compound name, and activity values based on literature studies. In the TIC figure, the x-axis (horizontal line) represents retention time, and the y-axis (vertical line) represents signal intensity or relative signal intensity. RT is the time required by a sample component of a compound to pass through the column to the detector. The measured mass is the mass obtained from the identified compound. This value is utilized to determine the molecular formula and name of the compound obtained. The metabolite profiling results on a 96% ethanol extract revealed a total of 61 compounds, including 43 known and 18 unknown compounds. The n-hexane fraction revealed a total of 64 compounds, including 49 known compounds and 15 unknown compounds. The ethyl acetate fraction showed 54 compounds, including 44 known and 10 unknown compounds. The butanol fraction revealed a total of 44 compounds, including 33 known compounds and 11 unknown compounds.
The metabolite profiling results revealed 208 metabolites throughout the l extract and fractions, including 160 known and 48 unknown compounds. According to the metabolite profiling, the peak retention time of the compound ranged from 0 to 14 minutes. Typically, peaks with RTs greater than 14 minutes are impurity peaks, such as those arising from solvent blanks or degradants, and are thus ignored. In separating, identifying, and calculating compounds in a mixture of compounds, RT is a crucial parameter. RT is affected by a variety of variables, including instrument specifications. The longer the reading time, the more fluctuations in system pressure and temperature the instrument will experience, which can affect the results of sample readings. To obtain optimal results, the Retention Time Locked (RTL) approach is utilized to eliminate sample reading variances that are restricted to RT 14 minutes52. As seen by the existence of unidentified chemicals in each extract and fraction, not all peaks in TIC can be recognized in the metabolite profiling technique utilizing the total number of detected metabolites. Compounds that cannot be found in the database are unidentified. These compounds could be impurities or degradation products that are still picked up by the instrument or new compounds that have not been recorded in the database, mainly unknown compounds with high concentrations53,54.
The analysis of these metabolites reveals the presence of several dominant or principal compounds, i.e., those with higher concentrations (as indicated by percentage area) than other compounds in the sample. In the 96% ethanol extract, the major compounds were N, N-Dimethyl-N’-1H-tettetrazoleylsulfuric diamide with a percentage area of 14.9591%, Dihomo-linolenic acid in the n-hexane fraction with a percentage area of 7.5074%, N, N-Dimethyl-N’-1H-tetrazole-5-ylsulfuric diamide in the ethyl acetate fraction with a percent area of 15.9989%, and {[2-(3-Methoxy-2-propoxypheneethyl]imino}di-2,1-ethanediyl bis (butylcarbamate) in the butanol fraction with a percentage area of 14.0886%.
M. mammosa tuber extract and fractions contain the antimicrobial compound Curcumene25. 2-Amino-6-chloroquinedioxolane is reported to have antiviral activity24. Scopoletin, 3-Hydroxycoumarin, Pyrogallol, Carboline, Curcumol, Ethyl 9H-carboline-3-carboxylate, 3-BHA, and Cynarine are antioxidant compounds22,32-34,37,42,47. By increasing the activity of SOD and glutathione transferase, the antioxidant activity reduces the ROS that can cause cell damage17. Naphthylamine and Pyocyanin are both antibacterial compounds 23,44. This antibacterial activity inhibits bacterial cell reproduction17. Scopoletin, Dihomo—linolenic acid, and Merremoside-D are anti-inflammatory compounds14,22,46. Anti-inflammatory drugs are crucial for surviving the cytokine storm, a severe inflammation caused by respiratory viral infections. Resin glycoside chemicals (merremoside) have anti-inflammatory activity; thus, it is expected that a synergistic effect will be established from the components in the M. mammosa tuber in conquering the SARS-CoV2 infection. In its application, multicomponent medicinal plants may be utilized as extracts or active fractions. Typically, plant fractions possess more excellent antiviral activity than pure substances, as medicinal plants typically include multiple physiologically active chemicals. To establish the potential of this activity, however, additional studies are required.
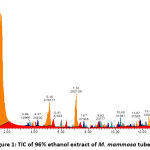 |
Figure 1: TIC of 96% ethanol extract of M. mammosa tuber |
 |
Table 1: Prediction of compounds in 96% ethanol extract of M. mammosa tuber |
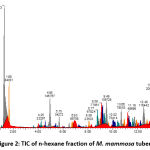 |
Figure 2: TIC of n-hexane fraction of M. mammosa tuber |
 |
Table 2: Prediction of compounds in n-hexane fraction of M. mammosa tuber |
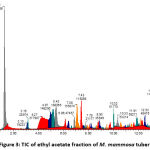 |
Figure 3: TIC of ethyl acetate fraction of M. mammosa tuber |
 |
Table 3: Prediction of compounds in ethyl acetate fraction of M. mammosa tuber |
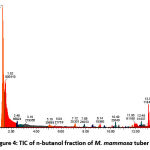 |
Figure 4: TIC of n-butanol fraction of M. mammosa tuber |
Table 4: Prediction of compounds in an n-butanol fraction of M. mammosa tuber
| No. | RT | %Area | Measured Mass | Molecular Formula | Compound Name | Activity |
| 1 | 2.462 | 4.0288 | 165.0789 | C9H11NO2 | D-Phenylalanine | Reduce pain (analgesic) 31. |
| 2 | 2.688 | 8.3197 | 141.1147 | Unknown | Unknown | – |
| 3 | 5.098 | 3.0715 | 516.1279 | C26H28O7S2 | 2-{[2-(4-Hydroxyphenyl)ethyl] sulfanyl}-3-[4-(2-{4-[(methylsulfonyl)oxy]phenoxy} ethyl)phenyl]propanoic acid | – |
| 4 | 5.500 | 1.8704 | 516.1265 | C25H24O12 | Cynarine | Choleretic and cholesterol-lowering, hepatoprotective, anti-atherosclerotic, anti-HIV, antioxidative, anti-diabetic, anti-carcinogenic, and immune modulator activity 47. |
| 5 | 6.421 | 0.3941 | 387.2380 | C19H29N7O2 | 7-[3-(3,4-Dihydro-2H-pyrrol- 5-ylamino)propyl]-1,3-dimethyl- 8-(1-piperidinyl)-3,7-dihydro-1 H-purine-2,6-dione | – |
| 6 | 6.856 | 0.0909 | 683.3510 | C39H49N5O4S | 7-[4-(2-Butoxyethoxy)phenyl]- 1-isobutyl-N-(4-{[(4-propyl-4H -1,2,4-triazol-3-yl)methyl]sulfinyl} phenyl)-2,3-dihydro-1H-1- benzazepine-4-carboxamide | – |
| 7 | 7.124 | 2.1770 | 308.0815 | C16H17O4Cl | 5-(4-Chlorophenoxy)pentyl 2-furoate | – |
| 8 | 7.341 | 0.0763 | 752.4034 | C45H56N2O8 | 3-Buten-1-yl [6a-(allyloxy)- 4-(ethoxyimino)-10-hydroxy-1,2- bis(4-hydroxybutyl)-1,2,4,5,6,6a,11b, 11c-octahydrobenzo[kl]xanthen-6-yl] (1-naphthylmethyl)carbamate | – |
| 9 | 7.496 | 0.1773 | 395.1516 | C21H22N5OCl | 1-(4-Chlorophenyl)-3-[4- (4-methyl-1-piperazinyl)-6-quinolinyl]urea | – |
| 10 | 7.890 | 2.0695 | 270.0654 | C8H16N4O2Cl2 | N-[(4,5-Dimethyl-4H-1,2,4- triazol-3-yl)methyl]-N-methylglycinedihydrochloride | – |
| 11 | 8.086 | 0.1587 | 395.1534 | C19H21N7OS | 8-Benzyl-N-(2-methoxyethyl)-6,7, 8,9-tetrahydropyrido[4′,3′:4,5] thieno[3,2-e]tetrazolo[1,5-a] pyrimidin-5-amine | – |
| 12 | 8.312 | 0.0715 | 254.0684 | C6H14N4O5S | Sulfuric acid | – |
| 13 | 8.572 | 1.7961 | 1086.5399 | C55H70N14O10 | D-Phenylalanyl-L-glutaminyl-N- {2-[1-(2-{[(2S)-1-{[(2S)-1-{[(2S)-1- amino-4-methyl-1-oxo-2-pentanyl] amino}-4-methyl-1-oxo-2-pentanyl] amino}-3-(1H-imidazol-5-yl) -1-oxo-2-propanyl]amino}-2-oxoethyl) -2,4-d ioxo-1,4-dihydro-3(2H)-chinazolinyl]ethyl}- L-tryptophanamid | – |
| 14 | 8.726 | 0.5247 | 393.1576 | C23H23NO5 | N-[4-(Benzyloxy)phenyl]-3,4,5- trimethoxybenzamide | – |
| 15 | 8.860 | 0.0330 | 924.4895 | C30H64N22O10S | Unknown | – |
| 16 | 9.057 | 0.7997 | 379.1555 | C20H26NO4Cl | DL-Norlaudanosine Hydrochloride | – |
| 17 | 9.141 | 1.6330 | 332.0794 | C19H12N2O4 | 2-Benzyl-5-nitro-benzo[de]isoquinoline- 1,3-dione | – |
| 18 | 9.492 | 0.8726 | 288.0882 | C12H17N2O4Cl | Methyl 4-[(N,N-dimethylglycyl) amino]-2-hydroxybenzoate hydrochloride (1:1) | – |
| 19 | 9.605 | 0.1566 | 268.1202 | C11H17N6Cl | 4-(1-Chloroethyl)-6-(3,5,5-trimethyl-4,5-dihydro-1H-pyrazol-1-yl)-1,3,5-triazin-2-amine | – |
| 20 | 9.823 | 0.0995 | 639.3743 | C33H49N7O6 | N-{(2R)-2-Cyclohexyl-2-[(2- pyrazinylcarbonyl)amino]acetyl}-3- methyl-D-valyl-N-[(3S)-1- (cyclopropylamino)-1,2-dioxo-3- hexanyl]-L-prolinamide | – |
| 21 | 9.915 | 0.1437 | 342.2879 | C19H38N2O3 | Lauramidopropylbetaine | – |
| 22 | 10.112 | 1.0649 | 477.3323 | C10H39N17O5 | Unknown | – |
| 23 | 10.245 | 0.7078 | 231.1067 | C5H17N3O7 | Unknown | – |
| 24 | 10.400 | 2.5244 | 423.3113 | C24H37N7 | 6-[(4-Amino-2-methyl-6- quinolinyl)amino]-2-{[(2S)-5- (diethylammonio)-2- pentanyl]amino}-4- methylpyrimidin-1-ium | – |
| 25 | 10.660 | 1.3237 | 355.0953 | C13H17N5O5S | 5′-S-(2-Carboxyethyl)-5′ -thioadenosine | – |
| 26 | 10.815 | 0.0645 | 1086.5440 | C47H78N10O19 | L-Lysyl-L-leucyl-L- threonyl-L-prolyl-L-α-glutamyl- L-α-glutamyl-L-α-glutamyl- L-α-glutamyl-L-isoleucine | – |
| 27 | 10.969 | 0.3587 | 311.1066 | C11H22N3O3SCl | 4-(5-Chloropentanoyl) -N,N-dimethyl-1-piperazinesulfonamide | – |
| 28 | 11.187 | 0.6646 | 981.5404 | C39H71N19O9S | Unknown | – |
| 29 | 11.405 | 0.5699 | 317.2919 | Unknown | Unknown | – |
| 30 | 11.560 | 0.3775 | 981.5510 | C45H71N15O10 | L-Histidyl-D-norleucyl-N5-(diaminomethylene)- L-ornithyl-L-tryptophylglycyl-L-lysyl- L-seryl-L-valine | – |
| 31 | 11.714 | 1.6791 | 531.3806 | C15H41N21O | Unknown | – |
| 32 | 11.953 | 5.2623 | 960.5294 | C48H80O19 | Notoginsenoside G | Immunological adjuvant activity and
immunostimulatory activity 29. |
| 33 | 12.376 | 0.8426 | 974.5444 | C39H74N16O11S | N5-(Diaminomethylen)- L-ornithyl-N5-(diaminomethylen)- L-ornithyl-L-threonyl-L-alanyl- L-leucyl-L-glutaminyl- L-cysteinyl-L-lysin | – |
| 34 | 12.459 | 2.6705 | 519.3336 | C28H46N5O2Cl | N4-(5-Chloro-2,4-dimethoxyphenyl) -N6-hexadecyl-4,5,6- pyrimidinetriamine | – |
| 35 | 12.656 | 2.4303 | 1009.5794 | C58H75N9O7 | N-{3-[18-(2-Carboxyethyl)-3,7, 12,17-tetramethyl -8,13-divinyl-2-porphyrinyl]propanoyl} leucyl-N-(11-methoxy-11-oxoundecyl) histidinamide | – |
| 36 | 12.924 | 0.3246 | 995.5600 | C45H73N17O7S | Unknown | – |
| 37 | 13.058 | 10.1941 | 495.3309 | C26H45N3O6 | {[2-(3-Methoxy-2-propoxyphenyl) ethyl]imino}di-2,1-ethanediyl bis (butylcarbamate) | – |
| 38 | 13.317 | 0.2499 | 278.1501 | C5H18N12S | Unknown | – |
| 39 | 13.409 | 0.1162 | 521.3465 | C28H47N3O6 | N’-[(Z)-(2,3,4-Trimethoxy-6-nitrophenyl) methylene] octadecanehydrazide | – |
| 40 | 13.535 | 0.7638 | 1009.5877 | C55H83N3O14 | 2-{[(1R,2R,4R)-4-{(1E)-2-[(1R,9S,12S,13R,14S,17 R,18E,21S,23S,24 R,25S,27R) -17-Ethyl-1,14-dihydroxy -23,25-dimethoxy-13,19,21,27- tetramethyl-2,3,10,16-tetraoxo -11,28-dioxa-4-azatricyclo[22.3.1.04,9] oct acos-18-en-12-yl]-1-propen-1-yl} -2-methoxycyclohexyl]oxy} -N-[4-(4-morpholinyl) phenyl]acetamid | – |
| 41 | 13.803 | 4.6668 | 509.3477 | C28H43N7O2 | 7,8-Dimethyl-3-[( {2-methyl-1-[1-(2-methyl-2-butanyl) -1H-tetrazol-5-yl]propyl} [2-(4-morpholinyl)ethyl]amino) methyl]-2(1H)-quinolinone | – |
| 42 | 14.020 | 0.1850 | 843.6769 | C34H81N23S | Unknown | – |
| 43 | 14.217 | 2.0541 | 523.3646 | C22H45N13S | Unknown | – |
| 44 | 14.527 | 12.5480 | 523.3635 | C29H45N7O2 | (2E)-2-{4-[3-(Diethylamino)propoxy] benzylidene}-N’-[(E) -{4-[3-(diethylamino)propoxy] phenyl}methylene] hydrazinecarbohydrazonamide | – |
Antioxidant phenolic substances such as scopoletin and 3-Hydroxycoumarin can be expected based on the results. Furthermore, glycoside molecules such as merremosida-D and Notoginsenoside G were discovered in the ethyl acetate and butanol fractions. In the ethyl acetate fraction, flavonoid compounds such as Gemixanthone A and terpene compounds like Curcumene were found.
Conclusion
M. mammosa tuber extract and fractions have different metabolite profiles. The ethanol extract yielded 61 chemicals, with N, N-Dimethyl-N’-1H-tetrazol-5-ylsulfuric diamide being the most abundant. The n-hexane fraction produced 64 molecules, the most abundant of which was dihomo-linolenic acid. The ethyl acetate fraction yielded 54 compounds, with N, N-Dimethyl-N’-1H-tetrazole-5-ylsulfuric diamide being the most abundant. The butanol fraction yielded 44 compounds, the most abundant of which was [2-(3-Methoxy-2-propoxyphenyl)ethyl]iminodi-2,1-ethanediyl bis (butylcarbamate). Several substances may have antiviral, antioxidant, anti-inflammatory, and other effects; thus, additional studies are required to determine the extent of these actions.
Acknowledgements
This research is funded by Penelitian Unggulan Fakultas Farmasi Universitas Airlangga 2022 with contract number 545/UN3.15/PT/202
Conflict of Interest
All authors declare there is no conflict of interest in this manuscript.
References
- Susilo A, Rumende C.M, Pitoyo C.W, Santoso W.D, Yulianti M, Sinto R, et al. Coronavirus disease 2019: Review of current literature. JurnalPenyakitDalam Indonesia, 2019; 7(1): 45-67.
- World Health Organization (WHO). WHO Coronavirus (COVID-19). Available at https://covid19.who.int/. accessed on 3 August 2022.
- Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care, 2020; 24(1): 1-4.
- Pandey S.C, Pande V, Sati D, Upreti S, Samant M. Vaccination strategies to combat novel coronavirus SARS-CoV-2. Life Sciences, 2020; 256: 1-9.
- Dewi F.R, Pratama I.P. Profil Efek Samping Obat Antiviral Pada Pasien Covid Di Rumah Sakit X Bali PeriodeTahun 2020. Jurnal Riset Kesehatan Nasional, 2021; 5(2): 137-149.
- Rusdi M. Mini Review: Farmakologipada Corona Virus Disease (Covid-19). Jurnal Ilmu Kefarmasian, 2021; 2(1): 54-61.
- Ang L, Lee H.W, Choi J.Y, Zhang J, Lee M. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res., 2020; 9(2): 1-14.
- Syamsu R.F, Nuryanti S, Arafah, Jama M.F. Herbal yang berpotensi sebagai antivirus pada Covid-19. Molucca Medica, 2021: 14(1): 76-85.
- Nahak M.P.M, Putri S.I, Rofiq Z, Purwanti W.P, Yunita A, Duarsa A.B.S, Fajriah A.S, Widiyanto A, Atmojo J. The use of herbs in facing Covid-19 pandemic: A systematic review. Avicenna: Journal of Health Research, 2022; 5(1): 37-49.
- Yang Y, Islam M.S, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int J Biol Sci., 2020; 16(10): 1708–1717.
- Feng J, Fang B, Zhou D, Wang J, Zou D, Yu G, et al. Clinical effect of traditional chinese medicine shenhuang granule in critically ill patients with covid-19: A single-centered, retrospective, observational study. J Microbiol Biotechnol., 2021; 31(3): 380-386.
- Wulandari A, Soleha S. Pharmacological activities of Merremia mammosa. Infokes, 2021; 11(1): 394-399
- Sharif E.U, Wang H.L, Akhmedov N.G, O’Doherty G. Merremoside D: De novo synthesis of the purported structure, NMR analysis, comparison of spectral data. Org Lett National Institutes of Health, 2014; 16(2): 492-495.
- Agil M, Studiawan H, Purwitasari N. Efficacy of Merremia mammosa Hall terpenoid fraction against Mycobacterium tuberculosis. Research J Pharm and Tech., 2021; 14(12): 6617-6620.
- Putri G.T.A, Sakinah E. The effect of bidaraupas tuber (Merremia mammosa (Lour.) Hailler f.) extract water fraction toward collagen density in diabetic model rat wounds. Journal of Medicinal Plant Indonesia, 2020; 13(1): 41-49.
- Nile S.H, Keum Y.S, Nile A.S, Jalde S.S, Patel R. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol., 2017; 32(1): 1-8.
- Sakinah E, Ulfa E, Manti A. The effectiveness of Merremia mammosa (Lour.) extract fractions as diabetic wound healers on diabetic rat model. Hiroshima J Med Sci., 2018; 67: 70-77.
- Hidayat F, Elfiah U, Sofiana K. Comparison of the number of macrophage in full thickness wound incision between Merremia mammosa extract treatment and NaCl in male wistar rats. Journal of Agromedicine and Medical Sciences, 2015; 1(1): 9-13.
- Gowda G.N, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Review of Molecular Diagnostics, 2014; 8(5): 617–633
- Naushad M, Khan M. Ultra performance liquid chromatography mass spectrometry: Evaluation and applications in food analysis. CRC Press, New York. 2014
- Zhang Z, Bo T, Bai Y, Ye M, An R, Cheng F, Liu H. Quadrupole time-of-flight mass spectrometry as a powerful tool for demystifying traditional Chinese medicine. Trends in Analytical Chemistry, 2015; 72: 169-180
- Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand J, Heim CE, et al,. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host & Microbe, 2017; 22(1): 25-37.
- Firmansyah A, Winingsih W, Manobi J.D. Review of Scopoletin: Isolation, Analysis Process, and Pharmacological Activity. Biointerface Research in Applied Chemistry, 2021; 11(4): 12006-12019.
- Petrikaite V, Tarasevicius E, Pavilonis A. Synthesis and Antimicrobial Properties of Naphthylamine Derivatives Having a Thiazolidinone Moiety. Medicina, 2011; 47(6): 334-9
- Zhou L, Coats SJ, Zhang H, Junxing S, Bobeck D.R, Schinazi R. Scaleable processes for the synthesis of (-)-β-D-2,6-diaminopurine dioxolane (Amdoxovir, DAPD) and (-)-β-D-2-aminopurine dioxolane (APD). Tetrahedron, 2012; 68(29): 5738-5743.
- Silva G.N.S.D, Pozzatti P, Rigatti F, Hörner R, Alves S.H, Mallmann C.A, Heinzmann B. Antimicrobial evaluation of sesquiterpene alpha-curcumene and its synergism with imipenem. J Microbiol Biotech Food Sci., 2021; 4(5): 434-436
- Chen C.Y, Chiu F.Y, Lin Y, Huang W.J, Hsieh P.S, Hsu F. Chemical constituents analysis and anti-diabetic activity validation of four fern species from Taiwan. Intl J M Sci., 2015; 16(2): 2497-2516.
- Dmitrenko D, Shnayder N, Kiselev I, Shulmin A, Zhirova N, Shapovalova E, Kantimirova E, Bochanova E, Veselova O, Panina Y, Muravieva A. Problems of rational therapy for epilepsy during pregnancy. Open Journal of Obstetrics and Gynecology, 2014; 4(9): 506-515
- Fung D, Whitson J. An evidence-based review of unoprostone isopropyl ophthalmic solution 0.15% for glaucoma: Place in therapy. Clin Ophthalmol., 2014; 8: 543-554
- Liu H, Lu X, Hu Y, Fan X. Chemical constituents of Panax ginseng and Panaxnotoginseng explain why they differ in therapeutic efficacy. Pharmacol Res., 2020; 161: 1-17.
- Sales-Campos H, Souza P, Peghini C.B, Silva S.J, Cardoso R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem., 2013; 13(2): 201-210.
- Perna S, Alalwan T.A, Al-Thawadi S, Negro M, Parimbelli M, Cerullo G, Gasparri C, et al. Evidence-based role of nutrients and antioxidants for chronic pain management in musculoskeletal frailty and sarcopenia in aging. Geriatrics, 2020; 5(1): 1-12
- Torres F.C, Brucker N, Andrade S.F, Kawano D.F, Garcia S.C, Poser G.L, Eifler-Lima V. New insights into the chemistry and antioxidant activity of coumarins. Curr Top Med Chem., 2014; 14(22): 2600-2623
- Sutanto H, Susanto B.H, Nasikin M. Solubility and antioxidant potential of a pyrogallol derivative for biodiesel additive. Molecules, 2019; 24(13): 1-16.
- Li S.P, Wang Y.W, Qi S.L, Zhang Y.P, Deng G, Ding W.Z, Ma C, Lin Q.Y, Guan H.D, Liu W, Cheng X.M, Wang C. Analogous β-Carboline alkaloids harmaline and harmine ameliorate scopolamine-induced cognition dysfunction by attenuating acetylcholinesterase activity, oxidative stress, and inflammation in mice. Front Pharmacol., 2018; 9(346): 1-16.
- Choi D.S, Ji Y, Jang Y, Lee W.J, Shim W. Crotamiton, an Anti-Scabies Agent, Suppresses Histamine- and Chloroquine-Induced Itch Pathways in Sensory Neurons and Alleviates Scratching in Mice. Biomol Ther (Seoul)., 2020; 28(6): 569-575.
- Marsh W. Propylhexedrine. xPharm: The Comprehensive Pharmacology Reference. Elsevier, 2007; 1-4
- Hashem S, Nisar S, Sageena G, Macha M.A, Yadav S, Krishnankutty R, Uddin, et al,. Therapeutic effects of curcumol in several diseases; An overview. Nutr Cancer, 2021; 73(2): 181-195.
- Pangal A, Ahmed K, Shaikh S. Synthesis, characterization and study of antimicrobial activity of 2,6-Ditertiary butyl-1,4-benzoquinone hydrazones. Int Res J Pharm., 2013; 4(8): 172-176
- Teimuri-Mofrad R, Rahimpour K, Poursadegh A. Investigation of 5,6-dimethoxy-1-indanone derivatives in optical applications: Synthesis, characterization and study of linear and nonlinear optical properties. Materials Chemistry and Physics, 2017; 200: 384-394.
- Jamshidfar S, Ardakani Y.H, Lavasani H, Rouini M. Inhibition of mirtazapine metabolism by Ecstasy (MDMA) in isolated perfused rat liver model. Daru Journal of Pharmaceutical Sciences, 2017; 25(16): 1-7.
- Kwon O.S, Park H.R, Yun B.S, Hwang J.H, Lee J.C, Park D.J, Kim C. Antimicrobial activity of N-acetyl-phenylalanine produced from Streptomyces sp. G91353. Korean Journal of Microbiology and Biotechnology, 2006; 34: 306-310
- Yehye W.A, Rahman N.A, Alhadi A.A, Khaledi H, Ng S.W, Ariffin A. Butylatedhydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules, 2012; 17(7): 7645-7665
- Chen-Hussey V, Behrens R, Logan J. Assessment of methods used to determine the safety of the topical insect repellent N,N-diethyl-m-toluamide (DEET). Parasites & Vectors, 2014; 7(1): 1-7.
- Marrez D.A, Mohamad H. Biological activity and applications of Pyocyanin produced by Pseudomonas aeruginosa. Open Acc J Bio Sci., 2020; 2(1): 140-144
- Committee for Medicinal Products for Human Use (CHMP). Guideline on the use of phthalates as excipients in human medicinal products. European Medicines Agency, London. 2012
- Wang X, Lin H, Gu Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis., 2012; 11(25): 1-9.
- Gazer C. Potential health effects of the popular compound of artichoke: Cynarin (CYN). ProgrNutr, 2017; 19(1): 5-9.
- Lu J, Muhmood A, Czekała W, Mazurkiewicz J, Dach J, Dong R. Untargeted metabolite profiling for screening bioactive compounds in digestate of manure under anaerobic digestion. Water, 2019; 11(11): 1-12
- Divyangini T, Desai DC, Namrata G, Bhumika G, Narkhede S. A review on solid phase extraction: 96-well spe plates format. European Journal of Biomedical and Pharmaceutical Sciences, 2018; 5(5): 267-271.
- Simpson J. Solid-Phase Extraction: Principles, Techniques, and Applications. New York: CRC. Press. 2000.
- Hajeb P, Zhu L, Bossi R, Vorkamp K. Sample preparation techniques for suspect and non-target screening of emerging contaminants. Chemosphere, 2002; 287(3): 1-17.
- Tyagi R, Elfawal M.A, Wildman S.A, Helander J, Bulman C.A, Sakanari J, et al. Identification of small molecule enzyme inhibitors as broad-spectrum anthelmintics. Sci Rep., 2019; 9(1): 1-13.
- Ma’arif B, Mirza D.M, Suryadinata A, Muchlisin M. Agil M. Metabolite profiling of 96% ethanol extract from Marsilea crenata Presl. leaves using UPLC-QToF-MS/MS and anti-neuroinflammatory predicition activity with molecular docking. J Trop Pharm Chem., 2019; 4(6): 261-270.
- Aditama A.P.R, Ma’arif B, Mirza D.M, Laswati H, Agil M. In vitro and in silico analysis on the bone formation activity of n-hexane fraction of semanggi (Marsilea crenata ). Sys Rev Pharm., 2020; 11(11): 837-849.








