Aiman Q. Al-Maathidy1*,Fardous Karawya,1, 2 Samer Y. Al-Qaraleh1 and Aiman Al-Qtaitat1, 3
1Department of Anatomy and Histology, Faculty of Medicine, University of Mutah, Al-Karak, Jordan.
2Department of Anatomy and Histology, Faculty of Medicine, University of Mutah and Faculty of Medicine, University of Alexandria, Alexandria, Egypt
3Department of Anatomy and Histology, Faculty of Dentistry, University of Zarqa, Al Zarqa, Jordan.
Corresponding Author E-mail: aimanafar@mutah.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2674
Abstract
Background: Phthalates are known to be major environmental hazards. Dibutyl phthalate (DBP), a commonly used phthalate ester, is present in a variety of products. Humans can be exposed to DBP from various sources, which can release it into biological fluids and cause various health problems by penetrating different tissues in the body. Aims: The aim of this study was to investigate the effects of DBP on pulmonary alveoli in rats and to assess the mitigating influence of S. platensis. Methods: The study involved 30 young adult male albino rats, which were divided into 3 groups (n = 10 each): control, group II (rats treated with phthalate ester (DBP; 50 mg/kg body weight/day)), and group III (Spirulina-protected animals given phthalate ester (DBP; 50 mg/kg body weight + Spirulina (200 mg/kg body weight/day)). Results: The study revealed that alveolar tissues in the groups treated with DBP showed significant increases in collagen deposition and inflammatory cellular infiltration. Furthermore, the numbers of type-II pneumocytes and alveolar macrophages were significantly increased. However, most of these effects were ameliorated by Spirulina platensis. Conclusion: These findings suggest that Spirulina may have potentially beneficial effects on pulmonary alveoli by mitigating the toxic effects of DBP.
Keywords
Dibutyl phthalate (DBP); Pulmonary alveolar type-II pneumocytes; Spirulina; Rats
Download this article as:| Copy the following to cite this article: Al-Maathidy A. Q, Karawya Q, Al-Qaraleh S. Y, Al-Qtaitat A. Histological Study of the Possible Protective Effect of Spirulina Platensis on Dibutyl Phthalate (DBP)-Induced Pulmonary Alveolar Changes in Adult Male Albino Rats. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Al-Maathidy A. Q, Karawya Q, Al-Qaraleh S. Y, Al-Qtaitat A. Histological Study of the Possible Protective Effect of Spirulina Platensis on Dibutyl Phthalate (DBP)-Induced Pulmonary Alveolar Changes in Adult Male Albino Rats. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3XDlx3Q |
Introduction
Environmental pollution by chemicals as a result of human activity constitutes a threat to human and animal health, and it has continued to attract attention globally. Chemical contamination by pollutants from food processing may occur through naturally-occurring contaminants, or as a result of interaction between food components and packaging materials during food processing and manufacturing, resulting in the migration of some toxic compounds into foods [1-5]. Although governments have been involved in essential steps to reduce hazardous pollutants in foods, there is need for extra efforts to reduce the health risks and diseases associated with chemical food contamination.
Endocrine-disrupting chemicals (EDCs) are a class of synthetic compounds that are very diverse, and they are used in different inductrial fields. In this area, insecticides, plasticizers (phthalates), and plastics (bisphenol A) are the most common chemicals that constitute threats to human health, and they have the potential to cause morbidity [6 – 12]. The use of plastics creates environmental problems not only because the bulk of junk produced is increased by phthalates, but they are also found in adhesives, paints, air freshener products, and personal care products. Dibutyl phthalate (DBP) is a common phthalate ester found in a variety of products such as food packaging, personal care products, children’s toys, and medical devices. When plastics are exposed to high temperatures, hazardous components are leached out, or are made to migrate into the environment in the form of microplastics (MPs) or nanoplastics. These microforms of plastics accumulate in food chains and become accessible to direct or indirect human exposure via eating, skin contact, and inhalation. Microplastics (MPs) are subdivided into two main groups: primary and secondary MPs. Primary MPs are used both in the industry as plastic pellets and in personal care products like toothpaste, nail varnish, sun creams, scrubs, and bath gels. In contrast, secondary MPs are produced from plastic excess that is dispersed into the environment, and are steadily degraded due to photo- and thermo-oxidative processes, as well as mechanical abrasion, resulting in their accumulation in the human body. Textile fibers and manufacturing packings discharged into washing water from machine-washed clothes are the principal sources of these litters [13-19].
Epidemiology-based studies have shown that exposure to chemical contaminant has been associated with a variety of health problems including hematotoxicity, neurotoxicity, cardiotoxicity, nephrotoxicity, hepatotoxicity, and endocrine disruption [9-11]. A functional endocrine system is required for normal growth, development, and maintenance. However, in recent years, many environmental pollutants such as phthalate esters have been shown to interfere with hormonal activity. Nutritional interventions for supporting optimal immune system function are frequently overlooked in public health. Majority of micronutrients have pleiotropic effects on immunological function. Hence, Spirulina, as a natural “functional food”, should be investigated regarding its potential to ameliorate nutritional shortages and protect the immune system [20-33].
Spirulina cyanobacteria belomgs to blue-green algae. This microorganism has been used as a source of protein and vitamins for people, without resulting in any noticeable side effects. It is already available in health food stores as a dietary supplement in a range of formulations and tablets or drinks. Moreover, it has been recognized as non-toxic in many toxicological investigations. Numerous studies on the efficacy and clinical use of Spirulina in management of many illnesses has suggested that this alga has antiviral, anticancer, and anti-allergic properties [34-42].
Acute respiratory tract infections are major causes of morbidity and mortality around the world. Nowadays, COVID-19 is the main cause of death locally and worldwide. Although, the mitigating influence of Spirulina has been examined in a variety of tissues, only few studies have investigated its benbeficial effects on pulmonary tissue. The present research was aimed at investigating the chronic toxicity of DBP in rat pulmonary alveoli, as well as the mitigating influence of S. platensis on the process.
Materials and Methods
Experimental design
A total of 30 adult male albino rats weighing 150 to 200 g, were used in this study. The rats were kept in a room in an environment with equal durations of light and darkness at 24 ± 1°C, and humidity of 50 ± 10 %. The study received approval from the ethical authority of our Medical Faculty. The animals were divided into three sets, each with 10 animals. Rats in control group comprised 2 equal sub-sets given either normal feed or S. plantensis (0.20 g/kg bw daily) [43]. Group II received dibutyl phthalate (DBP; 50 mg/kg bw/day), while the 3rd group was given S. platensis (0.20 g/kg bw daily), in addition to DBP (0.050 g/kg bw daily). Each treatment was administered for 2 months via gavaging. Dibutyl phthalate (DBP) was supplied by Sigma Company, St Louis, MO, USA. The blue-green alga was obtained in powder form from a local vendor. Daily body weights, as well as feed and water consumption, were recorded. Daily treatment doses were adjusted in line with body weight changes. The doses were chosen based on the overall no-observed-adverse-effect level (NOAEL) for DBP (50 mg/kg bw/day) which was reported by other researchers [44].
Anatomical studies
At designated times, the rats were euthanized using overdose of sodium pentobarbital (200 mg/kg). The lungs were excised and examined for any changes in shape or weight. The length of each lung was measured, from the most prominent point in the apex to a point in the middle of the basal surface, while the width was measured from the middle of the hilum to the point meeting this point horizontally on the sternocostal surface.The organs were weighed using mini scale. Then, the data were input into IBM SPSS 20.0 software. The results are presented as mean ± SD, i.e., minimum and maximum. Data with normal distribution were compared amongst multiple groups with analysis of varaince.
Tissue processing for microscopy
The right lung tissue was processed for microscopy using standard procedures. These involved tissue fixation in HCHO, clearing, dehydration, sectioning, H&E staining and routine examination under a light microscope. The other lung tissue was fixed in glutaraldehyde solution (3%), followed by processing for TEM. Electron microscopy was done, and photographs of relevant fields were obtained using Joel 100CX TEM [45].
Results
In this research, DBP administration for 8 weeks did not result in death or changes in appearance in any rat group, relative to control. No apparent morphological and anatomical changes were detected during dissection of the lung in any of the experimental groups, either in the color of the organ, or in its shape. Lung weight was within the normal range of 385-395 mg (for the right lung) and 265-272 mg (for the left lung) in all groups (Table 1). The average external length of the right lung was 2.8-3.4 cm, while that of the left lung was in the range of 1.8-2.3 cm. The average values of external width for the right and right lungs were 0.5-1-0 and 0.4-0.8 cm, respectively. As shown in Table 1, there were no significant differences in these parameters in left lung in all groups (positive, negative control, treated, protected; p > 0.05).
Table 1: Lung length, width, and weight in each group.
| Group | Right lung | Left lung | ||||
| Length | Width | Weight | Length | Width | Weight | |
| Group Ia | 3.07±0.23 | 0.77±0.19 | 390.45±3.30 | 2.02±0.17 | 0.60±0.14 | 267.65±3.15 |
| Group Ib* | 3.02±0.31 | 0.68±0.19 | 390.28±3.14 | 1.95±0.19 | 0.62±0.12 | 269.17±1.57 |
| Group II | 3.08±0.23 | 0.75±0.19 | 390.00±2.98 | 2.02±0.17 | 0.60±0.14 | 268.72±1.71 |
| Group III | 3.05±0.26 | 0.73±0.18 | 390.88±1.61 | 2.05±0.19 | 0.60±0.14 | 268.30±1.43 |
| F
P |
1.25
0.365 |
2.07
0.105 |
0.882
0.432 |
1.98
0.211 |
0.874
0.411 |
0.41
0.811 |
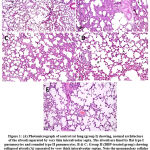
Light microscopy (H and E) results: Rat lung paraffin slices from controls (-ve and +ve) had essentially normal alveolar features (Figure 1 A). Relative to controls, group II (experimental group, i.e., DPB-treated), histological sections of rat right lungs of group II revealed presence of pulmonary lesions reflected in some collapsed alveoli and distended alveoli, thickened inter-alveolar septa due to cellular infiltration as well as hyaline material, proliferation of type II pneumocytes, foamy macrophages and congested blood vessels. There were desquamated cells inside the alveolar lumen (Figures 1 B and 1 C).
In group III (protected group): co-treatment with S. platensis and DBP produced significant protection of alveolar morphorlogy which is evident in mitigation of alveolar lesions, except for slight enlargement of inter-alveolar septae due to infiltration of cells associated with mild congestion of blood vessels. Several bloated alveoli were lined with more or less normal type I and II pneumocytes. A slight thickening appeared in the inter-alveolar septa due to collagen fibers and few cellular infiltrations. Moreover, the numbers of macrophages and septal cells were less than those in group II (Figures 1 D and 1 E).
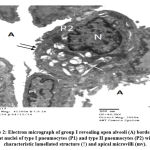
Data of TEM in +ve and -ve control rats revealed normal morphorlogy of alveoli with distended walls lined by type I pneumocytes and clear cytoplasm, and type II pneumocytes with apical microvilli facing alveolar lumen, indicating characteristic lamellated structure (Figure 2).
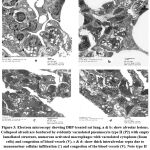
In DPB-treated rats, lung tissue showed collapsed alveoli demarcated with abnormal type II pneumocytes having empty lamellar bodies. Alveoli separation with thick interalveolar septa occurred due to infiltration by different cells: mononuclear inflammatory cells, septal cells or foamy macrophage, and desquamated cells in the lumen, in addion to significant increase in collagen deposition (Figures 3 and 4).
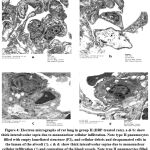 |
Figure 4: Electron micrographs of rat lung in group II (DBP-treated rats). a & b: show thick interalveolar septa due to mononuclear cellular infiltration. |
Group III (protected group) had considerable degree of preservation of the alveolar architecture which was evident in reduced alveolar lesions, although slight enlargement of inter-alveolar septa due to cell invasion associated with mild congestion of blood vessels. There were numerous swollen alveoli lined with more or less normal type I and II pneumocytes. The inter-alveolar septa appeared mildly thickened due to collagen fibers and few cellular infiltrations. The numbers of macrophages and septal cells were less than that in group II (Figure 5).
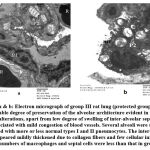 |
Figure 5 a & b: Electron micrograph of group III rat lung (protected group) revealed considerable degree of preservation of the alveolar architecture evident in decreased alveolar alterations, |
Discussion
The present study was aimed at elucidating the effect of chronic exposure of DBP on pulmonary alveoli, and the possible protective effect of Spirulina. The DBP treatment exacerbated alveolar injury. This may be attributed to oxidative stress which was morphologically reflected in proliferation of type II pneumocytes and alveolar macrophages, collapsed alveoli, cellular infiltrations, congestion of blood vessels and desquamated cells. It is generally agreed that nutrient deficiency may lead to changes in immunity which are demonstrated as changes in levels of T-cells, IgA antibody response, cytokines and NK cells [46-49].
Spirulina might modulate the immune system by mitigating nutritional deficiencies. Previous research have demonstrated that phycocyanin (PC), a protein extracted from cyanobacteria, has a variety of benefits such as antioxidant and anti-inflammatory properties [50-53]. Moreover, PC has been shown to reduce paraquat-induced lung damage in rats. In addition, in lipopolysaccharide-stimulated macrophages, PC, a selective cyclooxygenase-2 inhibitor, promotes apoptosis.These findings corroborate the findings in this study which showed reductions in macrophages and desquamated cells when Spirulina and DBP were given together. Furthermore, other studies revealed that Spirulina inhibited the release of histamine from mast cells. Indeed, analysis of blood samples of volunteers before and after oral administration of Spirulina platensis has shown that it has anti-inflammatory and antiviral properties[42,43]. Correspondingly, in managing patients with allergic rhinitis, Spirulina administration resulted in considerably ameliorated physical manifestations and symptoms such as nasal congestion, nasal discharge, sneezing, and itching [54-63]. Phthalate-mediated pulmonary lesions may precipitate fibrotic changes. Phthalates produce these delecterious changes in epithelial cells through complex molecular mechanisms mediated by oxidative insults which ultimately result in apoptotic lesions, generation of pro-fibrosis cytokines, and massive deposits of extracellular matrix. The end results of these processes is the establishment of pulmonary fibrosis. These are in agreement with the results obtained in this study, as is evident in presence of desquamated and exfoliated cells in the lumen of the alveoli, as well as increases in collagen fibers. Other study proposed that autophagy is an upstream event triggering lysosomal membrane permeabilization that would lead to apoptosis through the lysosomal-mitochondria pathway as described by others [40]. On the other hand, in vitro, an aqueous extract of Spirulina reduced HIV-1 multiplication in human T-cells, peripheral blood mononuclear cells, and Langerhans cells. Consequently, employing herbs and algae products with proven antiviral capabilities in combating specific viruses could be done through immunomodulation, even after the illness has been established [63-65]. These findings are consistent with those obtained in this study, as evidenced in reduction in cellular infiltration when Spirulina and DBP were given together. Of course, before any conclusions can be formed, these potential results must be further investigated in animal models and humans. However, the effect of Spirulina platensis only on one group of rats,, untreated with DBP, was not observed in this study. Therefore, this effect can be investigated in future studies of the same type of research.
It has been suggested by many researchers that the antioxidant and immuno-modulatory properties of Spirulina may have a tumor-destructive implication, thereby making it play a role in cancer prevention [64-66]. The anti-tumor potential of Spirulina are thought to be derived from β-carotene, a potent antioxidant. Nevertheless, the link between carotene contents and cancer cannot be proved because the pathogeneisis of cancer is typically complex. Currently, many studies have demonstrated the safety of Spirulina supplements, with few adverse effects, but its full potential as a medication is unknown.
Conclusion
Phthalates have a large scale of applications, and their usage is an extensive source of environmental pollution. Exposure to phthalates has been linked to poor pulmonary health, including lung fibrosis. Spirulina has a potential beneficial effect in mitigating the toxic effects of phthalates on the pulmonary alveoli. Thus, it is critical to educate the public on the dangers of phthalates so as to enhance their well-being. Therefore, the public must recognize the sources and pathways of phthalate exposure so that they significantly avoid them, particularly in those liable to exposure.
Conflict of Interest
there is no conflict of interest.
References
- Sergievich A.A., Khoroshikh P.P., Artemenko A.F., Zakharenko A.M., Chaika V.V., Kodintsev V.V., Stroeva O.A., Lenda E.G., Tsatsakis A., Burykina T.I., Agathokleous E., Kostoff R.N., Zlatian O., Docea A.O., Golokhvast K.S. Behavioral impacts of a mixture of six pesticides on rats. Total Environ. 2020; 727:138491.
- Docea A.O., Goumenou M., Calina D., Arsene A.L., Dragoi C.M., Gofita E., Pisoschi C.G., Zlatian O., Stivaktakis P.D., Nikolouzakis T.K., Kalogeraki A., Izotov B.N., Galateanu B., Hudita A., Calabrese E.J., Tsatsakis A. Adverse and hormetic effects in rats exposed for 12 months to low dose mixture of 13 chemicals: RLRS part III. Lett. 2019; 310:70–91.
- Tsatsakis A., Tyshko N.V., Docea A.O., Shestakova S.I., Sidorova Y.S., Petrov N.A., Zlatian O., Mach M., Hartung T., Tutelyan V.A. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes – a real-life risk simulation approach. Toxicol. Lett. 2019; 315:96–106.
- Warner, G.R.; A Flaws, J. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicol. Sci. 2018, 166, 246–249.
- Docea A.O., Calina D., Gofita E., Arsene A.L., Kouretas D., Tsatsarakis M. Effects of long-term low dose exposure to mixtures of pesticides, food additives and consumer products chemicals on biochemical parameters. Toxicol. Lett. 2017;280 S217-S.
- Tsatsakis A., Tyshko N.V., Docea A.O., Shestakova S.I., Sidorova Y.S., Petrov N.A., Zlatian O., Mach M., Hartung T., Tutelyan V.A. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes – a real-life risk simulation approach. Toxicol. Lett. 2019; 315:96–106.
- Zwierello, W.; Maruszewska, A.; Skorka-Majewicz, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Dec, K.; Styburski, D.; Nowakowska, A.; Gutowska, I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere 2020, 240, 124901.
- Shim, Y.H.; Ock, J.W.; Kim, Y.J.; Kim, Y.; Kim, S.Y.; Kang, D. Association between heavy metals, bisphenol a, volatile organic compounds and phthalates and metabolic syndrome. J. Environ. Res. Public Health2019, 16, 671.
- Warner, G.R.; A Flaws, J. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicol. Sci. 2018, 166, 246–249.
- SMargina D., Nițulescu G.M., Ungurianu A., Mesnage R., Goumenou M., Sarigiannis D.A. Overview of the effects of chemical mixtures with endocrine disrupting activity in the context of real-life risk simulation: an integrative approach (Review) World Academy of Sciences journal. 2019;1(4):157–164.
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Rev. Endocrinol.2019, 16, 45–57.
- Majeed, K.A.; Rehman, H.U.; Yousaf, M.S.; Zaneb, H.; Rabbani, I.; Tahir, S.K.; Rashid, M.A. Sub-chronic exposure to low concentration of dibutyl phthalate affects anthropometric parameters and markers of obesity in rats. Sci. Pollut. Res.2017, 24, 25462–25467.
- Margina, D.; Nițulescu, G.; Ungurianu, A.; Mesnage, R.; Goumenou, M.; Sarigiannis, D.; Aschner, M.; Spandidos, D.; Renieri, E.; Hernandez, A.; et al. Overview of the effects of chemical mixtures with endocrine disrupting activity in the context of real-life risk simulation (RLRS): An integrative approach (Review). World Acad. Sci. J.2019, 1, 157–164.
- Tsiaoussis J., Antoniou M.N., Koliarakis I., Mesnage R., Vardavas C.I., Izotov B.N., Psaroulaki A., Tsatsakis A. Effects of single and combined toxic exposures on the gut microbiome: current knowledge and future directions. Lett. 2019; 312:72–97.
- Sun, D.; Zhou, L.; Wang, S.; Liu, T.; Zhu, J.; Jia, Y.; Xu, J.; Chen, H.; Wang, Q.; Xu, F.; et al. Effect of Di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-thyroid axis in adolescent rat. J.2018, 65, 261–268.
- Petrakis D., Vassilopoulou L., Mamoulakis C., Psycharakis C., Anifantaki A., Sifakis S., Docea A.O., Tsiaoussis J., Makrigiannakis A., Tsatsakis A.M. Endocrine disruptors leading to obesity and related diseases. J. Environ. Res. Publ. Health. 2017;14(10):1282.
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Rev. Endocrinol.2019, 16, 45–57
- Margina, D.; Nițulescu, G.; Ungurianu, A.; Mesnage, R.; Goumenou, M.; Sarigiannis, D.; Aschner, M.; Spandidos, D.; Renieri, E.; Hernandez, A.; et al. Overview of the effects of chemical mixtures with endocrine disrupting activity in the context of real-life risk simulation (RLRS): An integrative approach (Review). World Acad. Sci. J.2019, 1, 157–164.
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine2017, 55, 666–681.
- Ogli, S.A.; Odeh, S.O.; Egesie, G.U. Gonadotoxic Potentials of Di- (2-Ethyl Hexyl) Phthalate in the Adult Male Wistar Rats. Res. Basic Clin. Sci.2019, 2, 163–171.
- Furr, J.R.; Lambright, C.S.; Wilson, V.S.; Foster, P.M.; Gray, L.E. A Short-term In Vivo Screen Using Fetal Testosterone Production, a Key Event in the Phthalate Adverse Outcome Pathway, to Predict Disruption of Sexual Differentiation. Sci.2014, 140, 403–424.
- Lu, X.; Xu, X.; Lin, Y.; Zhang, Y.; Huo, X. Phthalate exposure as a risk factor for hypertension. Sci. Pollut. Res. Int.2018, 25, 20550–20561.
- Park, J.; Park, C.; Gye, M.C.; Lee, Y. Assessment of endocrine-disrupting activities of alternative chemicals for bis(2-ethylhexyl) phthalate. Res.2019, 172, 10–17.
- Tsatsakis, A.M.; Katsikantami, I.; Kalantzi, O.; Sevim, Ç.; Tsarouhas, K.; Sarigiannis, D.; Tzatzarakis, M.N.; Rizos, A.K. Phthalates: Exposure and health effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Grand Rapids, MI, USA, 2019; pp. 163–173.
- Heacock, M.; Kelly, C.B.; Asante, K.A.; Birnbaum, L.S.; Bergman, A.L.; Brune, M.N.; Buka, I.; Carpenter, D.O.; Chen, A.; Huo, X.; et al. E-waste and harm to vulnerable populations: A growing global problem. Health Perspect.2016, 124, 550–555.
- Han, X.; Li, J.; Wang, Y.; Xu, S.; Li, Y.; Liu, H.; Zhou, Y.; Zhao, H.; Fang, J.; Cai, Z.; et al. Association between phthalate exposure and blood pressure during pregnancy. Environ. Saf.2020, 189, 109944.
- Xie, X.; Deng, T.; Duan, J.; Ding, S.; Yuan, J.; Chen, M. Comparing the effects of diethylhexyl phthalate and dibutyl phthalate exposure on hypertension in mice. Environ. Saf.2019, 174, 75–82.
- Deng, T.; Xie, X.; Duan, J.; Chen, M. Exposure to diisononyl phthalate induced an increase in blood pressure through activation of the ace/ at1r axis and inhibition of no production. Lett.2019, 309, 42–50.
- Su, T.C.; Hwang, J.S.; Torng, P.L.; Wu, C.; Lin, C.Y.; Sung, F.C. Phthalate exposure increases subclinical atherosclerosis in young population. Pollut.2019, 250, 586–593.
- Jaimes, R., 3rd; Swiercz, A.; Sherman, M.; Muselimyan, N.; Marvar, P.J.; Posnack, N.G. Plastics and cardiovascular health: Phthalates may disrupt heart rate variability and cardiovascular reactivity. J. Physiol. Heart Circ. Physiol.2017, 313, 1044–1053.
- Amara, I.; Timoumi, R.; Annabi, E.; Neffati, F.; Najjar, M.F.; Bouaziz, C.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate induces cardiac disorders in balb/c mice. Sci. Pollut. Res. Int.2019, 26, 7540–7549.
- Golestanzadeh, M.; Riahi, R.; Kelishadi, R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: A systematic review and meta-analysis. Sci. Pollut. Res. Int.2019, 26, 35670–35686.
- Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. 2020;12(6)
- Margina D., Ungurianu A., Purdel C., Tsoukalas D., Sarandi E., Thanasoula M. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. J. Environ. Res. Publ. Health. 2020;17(11)
- Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. 2020;12(4)
- Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. 2020; 12:236. doi: 10.3390/nu12010236.
- Zhou, L.; Chen, H.; Xu, Q.; Han, X.; Zhao, Y.; Song, X.; Zhao, T.; Ye, L. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Environ. Saf.2019, 170, 391–398.
- Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. HormBehav. 2019; 112:20–31. doi: 10.1016/j.yhbeh.2019.03.004
- Neyrinck MA, Taminiau B, Walgrave H, Daube G, Cani PD, Bindels LB, Delzenne N. Spirulina Protects against Hepatic Inflammation in aging: an effect related to the modulation of the gut microbiota? Nutrients. 2017;9(6):633. doi:10.3390/nu9060633.
- Pabon MM, Jernberg JN, Morganti J, Contreras J, Hudson CE, Klein RL. Bickford PC A spirulina-enhanced diet provides neuroprotection in an αsynuclein model of Parkinson’s disease, PLoS One. 2012;7(9): e45256.
- Palaniswamy R, Veluchamy C. Therapeutic uses of spirulina. International Journal of Current Innovation Research. 2018; 4:975–979.
- Patel S, Goyal A. Current and prospective insights on food and pharmaceutical applications of Spirulina. Curr. Trends Biotechnol. Pharm 2013; 7:95–681.
- Bhat VB, Madyastha KM. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA. BiochemBiophys Res Commun. (2001) 285:262–6. doi: 10.1006/bbrc.2001.5195
- Mylchreest E., Wallace D.G., Cattley R.C., Foster P.M.D. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Sci. 2000;55:143–151. doi: 10.1093/toxsci/55.1.143.
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. London: Churchill Livingstone; 2007. p. 125–13
- Greiller C., Martineau A. Modulation of the immune response to respiratory viruses by vitamin D. 2015; 7:4240–4270. doi: 10.3390/nu7064240.
- Maggini S., Pierre A., Calder P. Immune function and micronutrient requirements change over the life course. 2018; 10:1531. doi: 10.3390/nu10101531.
- Kalogeraki A., Stivaktakis P., Docea A., Calina D., Gofita E., Arsene A.L. Evaluation of long-term low dose exposure to mixtures on the lymphocytes of the peripheral blood of rats. Lett. 2017;280 S126-S.
- Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut Barking Essex, 20201987264:114732; doi: 10.1016/j.envpol.2020.114732.
- Ravi M, De SL. Azharuddin S and Paul S. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutrition and Dietary Supplements. 2010; 2:73–83.
- Neyrinck MA, Taminiau B, Walgrave H, Daube G, Cani PD, Bindels LB, Delzenne N. Spirulina Protects against Hepatic Inflammation in aging: an effect related to the modulation of the gut microbiota? Nutrients. 2017;9(6):633. doi:10.3390/nu9060633.
- Szulinska M, Gibas-Dorna M, Miller-Kasprzak E, Suliburska J, Miczke A, WalczakGałezewska M, Stelmach-Mardas M. Walkowiak J and Bogdanski P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: a randomized double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 2017; 21:2473–2481.
- Chakraborty B, Varsale AR, Singh VK, Mali SS, Parihar PK, Mane RS. Phytochemical analysis, antioxidant, and antifungal activity of different solvent extracts of Spirulina platensis collected from Rankala Lake, Kolhapur, Maharashtra. J. Algal Biomass Utln 2019; 10:36–42.
- Bashir MF, Ma BJ, Bilal null, Komal B, Bashir MA, Farooq TH, et al. 2020. Correlation between environmental pollution indicators and COVID-19 pandemic: A brief study in Californian context. Environ Res,2020; 187:109652
- Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol, 2020, 16:297–298; doi:10.1038/s41574-020-0353-9.
- El-Chaghaby GA, Rashad S, Abdel-Kader SF. Rawash EA and Abdul Moneem M. Assessment of phytochemical components, proximate composition and antioxidant properties of Scenedesmus obliquus, Chlorella vulgaris and Spirulina platensis algae extracts. Egyptian Journal of Aquatic Biology & Fisheries. 2019; 23:521–526.
- van’tErve, T.J.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Milne, G.L.; Calafat, A.M.; Swan, S.H.; Ferguson, K.K. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ. Sci. Technol. 2019, 53, 3258–3267.
- Wu, X.; Jiang, L.; Sun, X.; Yao, X.; Bai, Y.; Liu, X.; Liu, N.; Zhai, X.; Wang, S.; Yang, G. Mono(2-ethylhexyl) phthalate induces autophagy-dependent apoptosis through lysosomal-mitochondrial axis in human endothelial cells. Food Chem. Toxicol.2017, 106, 273–282.
- Kim H-M, Lee E-H, Cho H-H, Moon Y-H. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by Spirulina. Biochemical Pharmacology. 1998;55(7):1071–1076.
- O’Brien E et al. Perinatal bisphenol A exposure beginning before gestation enhances allergen sensitization, but not pulmonary inflammation, in adult mice. J Dev Orig Health Dis. 2014;5(2): 121–31.
- Gu, Z. W. et al. Expression and significance of T17 cells in nasal mucosa of allergic rhinitis mice. J. Clin. Pathol. Res. 36(11), 1776–1779 (2016).
- He, M. et al. Efects of airway exposure to di-(2-ethylhexyl) phthalate on allergic rhinitis. Immunopharmacol. Immunotoxicol. 35(3), 390–395 (2013)
- Liu, N.; Jiang, L.; Sun, X.; Yao, X.; Zhai, X.; Liu, X.; Wu, X.; Bai, Y.; Wang, S.; Yang, G. Mono-(2-ethylhexyl) phthalate induced ROS -dependent autophagic cell death in human vascular endothelial cells. Toxicol. In Vitro 2017, 44, 49–56.
- Hayashi K, Hayashi T, Kojima I. Hayashi K, et al. AIDS Res Hum A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities.1996Oct10;12(15):1463-71.doi: 0.1089/aid.1996.12.1463. AIDS Res Hum Retroviruses. 1996. PMID: 8893054
- Hwang, Y. H., Paik, M. J. & Yee, S. T. Diisononyl phthalate induces asthma via modulation of T1/T2 equilibrium. Toxicol. Lett. 272, 49–59 (2017).
- Nishioka J et al. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflamm Res. 2012;61(1):69–78