Nagham Mahmood Aljamali1* and Sabrean Farhan Jawad2
and Sabrean Farhan Jawad2
1Department of Chemistry ,Synthetic Organic Chemistry ,Iraq.
2Department of Pharmacy, Al-Mustaqbal University College, Babylon, Iraq.
Corresponding Author E-mail: dr.nagham_mj@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2296
Abstract
The importance of research lies in the treatment of cancerous tumors due to the spread of cancerous tumors in recent decades, so researchers have to insist on finding alternative and more treatments safe from chemotherapy and radiation, which are derivatives of some amino acids, which we attended in our current research. Also, some research showed that taking tryptophan for 3 days before exercise can improve energy and efficiency during exercise, but other preliminary research shows that taking tryptophan during exercise does not improve endurance during cycling exercises. For a few days before exercise to notice any benefit. In this research, we prepared derivatives of cyclic tryptophan and studied their efficacy as anti-tumors, and they gave good results in reducing the size of cancerous tumors and reducing their spread in the body., then sympathy all synthesized new cyclic-tryptophan compounds by numerous techniques (FT.IR ,H.NMR)–spectrophotometric, other physical and chemical properties ,with studying for one of new prepared derivatives as anti breast cancer.
Keywords
Anti Breast; Anticancer; Heterocyclic; Tryptophan; Triazole
Download this article as:| Copy the following to cite this article: Aljamali N. M, Jawad S. F. Preparation, Diagnosis and Evaluation of Cyclic-Tryptophan Derivatives as Anti Breast Cancer Agents. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Aljamali N. M, Jawad S. F. Preparation, Diagnosis and Evaluation of Cyclic-Tryptophan Derivatives as Anti Breast Cancer Agents. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3HuvlVG |
Introduction
In 1901, Frederick Hopkins was able to isolate tryptophan for the first time. Hopkins extracted tryptophan from hydrolyzed casein, successfully extracting (4-8 g) of tryptophan from (600 g) of crude casein[1,2]. Tryptophan is a less common amino acid in proteins, but it plays an important structural or functional roles wherever it is found. For example, the residues tryptophan and tyrosine play special roles in ‘fixing’ membrane proteins within the cell membrane. In addition, the functions of tryptophan include being a vital precursor to compounds3-5., Tryptophan stimulates serotonin levels in the brain, which can treat depression and anxiety. Therefore, you can take tryptophan supplements to reduce these indications 6-8. Tryptophan helps increase growth hormones, so it is essential for the proper growth of children and infants. Tryptophan is used to treat insomnia, due to the presence of serotonin, which is useful for controlling sleep patterns9-11. People who suffer from migraines are advised to take doses of tryptophan regularly, and to eat foods rich in tryptophan, which also helps prevent anxiety attacks, which improves a person’s reactions. It works to reduce appetite for food, because serotonin helps to make you feel satiated and reduce food intake. Thus, losing weight helps reduce diabetes and problems related to cardiovascular disease12-14. The body needs tryptophan to produce niacin, which helps generate good cholesterol and lower bad cholesterol. Foods rich in tryptophan also help convert carbohydrates into energy and maintain a healthy digestive system, skin, hair and eyes15-17., After we absorb tryptophan from food, our bodies convert it into 5-hydroxytryptophan, an amino acid that works in the brain and central nervous system by increasing the production of the chemical serotonin, and after our bodies convert it to this amino acid18-22, it then turns into serotonin, melatonin23-27 and Vitamin B6 (nicotinamide).
Some studies claim that tryptophan supplements may be effective as a sleep treatment and antidepressant. These findings are associated with its role in the synthesis of serotonin and melatonin28-30. Excessive encouragement of serotonin on postsynaptic (5-HT1A and 5-HT2A) receptors at the central and peripheral levels can have negative consequences for the organism31-34. This is known as serotonin syndrome and can be fatal. Although this syndrome can be caused by taking medications (eg, Prozac) or using drugs (eg, LSD, MDMA, methylphenidate, bath salts…), it is not likely to be caused by the consumption of nutritional supplements35-39.
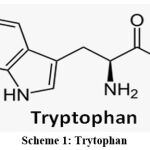 |
Scheme 1: Trytophan |
Experimental Part
Because of the importance of the prepared compounds in this research that they were studied as anti-cancer agents, we were keen to provide materials with high purity and from international companies with high technology in the production of chemicals. Also, the measurements were made on the following devices represented by ((FT-IR spectra (FT-IR 8300 Shimadzu) in the range (400-4000) cm-1 with KBr-discs., 1H.NMR–Spectra with (DMSO)–solvent .,besides to anti breast cancer studies.
Synthesis Progressions 8-12
Preparation Path of Compound {1}
Compound {1} formatted by reaction of tryptophan (0.01 mole) with thiosemicarbazide (0.01 mole) in presence of (5% of NaOH) with mechanical rotation for (16 hrs) in absolute ethanol permitting to mentioned methods8-12 to produce precipitation that acts compound{1},the last step ,filtered ,dried ,then recrystallized to give pure compound.
Preparation Path of Compound{2}
Compound {2} formatted by reaction of compound{1} (0.01 mole) with chloroacetoyl chloride (0.02 mole) in presence of (K2CO3) with mechanical rotation for (4 hrs) permitting to methods8-12 to yield precipitation that acts compound{2},the last step ,filtered ,dried ,then recrystallized to give pure compound.
Preparation Path of Compound{3}
Compound {3}cyclized by cyclization reaction of compound{2} (0.01 mole) with in presence of (K2CO3) with mechanical rotation and reflux for (6 hrs) permitting to methods8-12 to yield precipitation that acts compound{3},the last step ,filtered ,dried ,then recrystallized to give pure compound.
Preparation Path of Compound{4}
Compound {4} formatted by reaction of compound {3} (0.01 mole) with benzaldehyde (0.01 mole) in presence of (5% of NaOH) with mechanical rotation for (12 hrs) in absolute ethanol permitting to mentioned methods8-12 to produce precipitation that acts compound{4},the last step ,filtered ,dried ,then recrystallized to give pure compound.
Preparation Path of Compound{5}
Compound{5} formatted by reaction of compound{3} (0.01 mole) with m-nitrobenzaldehyde (0.01 mole) in presence of (5% of NaOH) with mechanical rotation for (13 hrs) in absolute ethanol permitting to mentioned methods8-12 to produce precipitation that acts compound{5},the last step ,filtered ,dried ,then recrystallized to give pure compound.
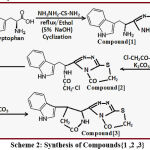 |
Scheme 2: Synthesis of Compounds{1 ,2 ,3} |
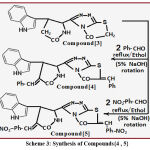 |
Scheme 3: Synthesis of Compounds{4 , 5} |
Results and Discussion
The prepared tryptophan-derivatives have been premeditated by different chemical performances and studies against cancerous cells:
Spectral Evidences of prepared Compounds
FT.IR- Investigation of Manufactured Derivatives
The bands of important groups in spectra provided strong evidences for new synthesized compound via disappearance of bands and appearance other new bands that point to formation of the new derivatives that represented by :
Compound {1}: appearance bands at (3282 , 3300)Cm-1 due to (NH2) of amine group, band at (3223)Cm-1 due to (NH) of amine group in triazole cycle, also at (2445)Cm-1 due to (SH) of thiol group, band at (1652)Cm-1 due to (C=N) of endocycle of triazole, at (2978)Cm-1 caused by (CH) aliphatic permitting to literature 16 .
Compound {2}: appearance band at (3369)Cm-1 due to (NH) of amine group in tryptophan, band at (1184)Cm-1 due to (S-CH2) of sulfide group, also at (1664)Cm-1 caused by (C=N) of endocycle of triazole, band at (2918)Cm-1 by reason of (CH) aliphatic, at (792)Cm-1 as a result of (C-Cl), band at (1681)Cm-1 due to (C-CO) amide group.
Compound {3}: appearance band at (3421)Cm-1 caused by (NH) of amine group in tryptophan, band at (1180)Cm-1 due to (S-CH2) of sulfide group, band at (1643)Cm-1 down to (C=N) of endocycle of triazole, band at (2954)Cm-1 due to (CH) aliphatic, band at (1664)Cm-1 down to (C-CO) amide group.
Compound {4}: appearance band at (3355)Cm-1 by reason of (NH) of amine group in tryptophan, band at (1148)Cm-1 due to (S-CH2) of sulfide group, band at (1655)Cm-1 due to (C=N) of endocycle of triazole, band at (2919)Cm-1 due to (CH) aliphatic, band at (1679)Cm-1 due to (C-CO) amide group, band at (3092)Cm-1 due to (CH=C) alkene , according to literature 16.
Compound {5}: appearance band at (3310)Cm-1 by reason of (NH) of amine group in tryptophan, band at (1133)Cm-1 down to (S-CH2) of sulfide group, at (1647)Cm-1 due to (C=N) of endocycle of triazole, band at (2906)Cm-1 down to (CH) aliphatic, band at (1682)Cm-1 down to (C-CO) amide group, band at (3097)Cm-1 down to (CH=C) alkene, bands at (1327 , 1511)Cm-1 due to (NO2) nitro group., Other frequencies appeared in some figures (1 , 2) .
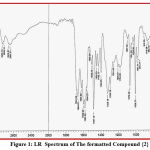 |
Figure 1: I.R Spectrum of The formatted Compound {2} |
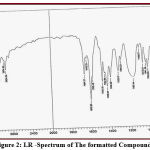 |
Figure 2: I.R -Spectrum of The formatted Compound {3} |
1H.NMR- Investigation of Manufactured Derivatives
The signals of important groups in spectra provided strong evidences for new synthesized compound via disappearance of peaks and appearance other new peaks that point to formation of the new derivatives that represented by : signals at (4.55 to 5.00) due to proton of amine group (NH) ,signals at (10. 12 to 10. 80) due to proton of amide (-NH-CO), signals at (6. 60-7. 95) due to protons of phenyl ring in compounds {2 ,3 , 4, 5}respectively, signal at (3.05) due to proton of (CO-CH2-S) in compounds {2 ,3}respectively , signal at (4.45) due to proton of (CH=C) of alkene in compounds {4 ,5}respectively permitting to literature (16) .,Some peaks in some figures (3, 4).
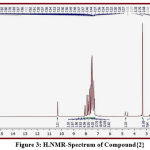 |
Figure 3: H.NMR-Spectrum of Compound{2} |
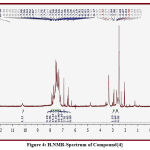 |
Figure 4: H.NMR-Spectrum of Compound{4} |
Some physical and Chemical characterization:
All other physical and chemical analysis besides to some description in Table (1):
Table 1: Some Physical Properties of New Compounds
| Comps | Product % | Color | M .P ( C ° ) | Rf | Solvents (TLC) |
| { 1 } | 82 | Orange | 160 | 0.64 | Ethanol : Hexane |
| { 2 } | 78 | Yellowish orange | 182 | 0.68 | Ethanol : Hexane |
| { 3 } | 70 | Deep Yellow | 194 | 0.64 | Ethanol : Hexane |
| { 4 } | 82 | Yellowish Red | 200 | 0.58 | Ethanol : Hexane |
| { 5 } | 80 | Bill Orange | 216 | 0.60 | Ethanol : Hexane |
Analysis of derivatives with Breast Cancer
The study was conducted to test the effectiveness of medicinal derivatives through the methods listed in the references17, 18 .
Table 2: Mean Percentage (%) for each cell line (Respond to Treatment) for Derivative{2}.
| Concentration of Tryptophan-Derivative [2]
/ ( µ g/Ml-1) |
MCF-7 | WRL | ||
| Mean | SD | Mean | SD | |
| 400 | 50.00 | 1.34 | 49.44 | 1.14 |
| 200 | 63.11 | 2.21 | 27.10 | 0.65 |
| 100 | 74.12 | 5.32 | 26.41 | 2.06 |
| 50 | 80.13 | 4.12 | 19.03 | 0.92 |
| 25 | 89.91 | 0.87 | 10.08 | 1.58 |
| 12.5 | 90.80 | 0.88 | 9.34 | 2.44 |
| 6.25 | 91.24 | 0.22 | 9.04 | 1.16 |
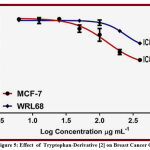 |
Figure 5: Effect of Tryptophan-Derivative [2] on Breast Cancer Cells |
Conclusion
The manufactured of Tryptophan-Derivative [2] gave multiple strong evidences about structures of compounds were stable, and all Tryptophan-Derivatives have high solubility in ethanol , DMSO, Methanol and other solvents. And have good activity against breast breast cancer cells.
Acknowledgment
we would like to express our heartfelt thanks to health-Lab for providing assistance samples of breast cancer for studying
Ethical clearance
Ethics committee refer that there is no plagiarism and there is no mistakes or wrong results in this work.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding source
There are no funding sources.
References
- Nagham Mahmood Aljamali., 2015. Synthesis and Chemical Identification of Macro Compounds of (Thiazol and Imidazol)”.,Research J. Pharm. and Tech, 8,1, 78-84., DOI: 10.5958/0974-360X.2015.00016.5.
CrossRef - Miaid Mohmed ,Nagham Mahmood Aljamali ,Sabreen Ali Abdalrahman., Wassan Ala Shubber., “Formation of Oxadiazole Derivatives Ligands from Condensation and Imination Reaction with References To Spectral Investigation, Thermal and Microbial Assay”., Biochem. Cell. Arch., 2018 ,18, 1, pp. 847-853.
- Pradhan, L.-P. Xu, S. Chen. Janus nanoparticles by interfacial engineering. Adv. Funct. Mater., 17 (14) (2007), pp. 2385-2392.
CrossRef - Nagham Mahmood Aljamali. 2016. “Synthesis and Biological Study of Hetero (Atoms and Cycles) Compounds”, Der Pharma Chemica, 8,6, 40-48.
- Thomas L. Gilchrist “Heterocyclic Chemistry” 3rd ed. Addison Wesley: Essex, England, 1997. 414 pp. ISBN 0-582-27843-0.
- Edon Vitaku, David T. Smith, Jon T. Njardarson (2014). “Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals”. J. Med. Chem. 57: 10257-10274. doi:1021/jm501100b
CrossRef - Wang, Cuiling; Yan, Jiaxu; Du, Mo; Burlison, Joseph A.; Li, Chi; Sun, Yanni; Zhao, Danqing; Liu, Jianli (2017). “One step synthesis of indirubins by reductive coupling of isatins with KBH4”. Tetrahedron . 73 (19): 2780–2785. doi:1016/j.tet.2017.03.077.
CrossRef - Nagham Mahmood Aljamali., “The Various Preparation Methods in Synthetic Chemistry”.,1 Edt. ,Evincepub Publishing house, 2019., ISBN :978-93-88277-82-2.
- Nagham Mahmood Aljamali, “Effect of Conditions and Catalysis on Products” .,1th –Edition, 2021 , Eliva Press SRL., ISBN: 9781636482286
- Nagham Mahmood Aljamali. “Reactions and Mechanisms”.,1 Edt., IJMRA Publication ,2018 .,ISBN : 978- 93-87176-25-6 .
- Nagham Mahmood Aljamali. “Experimental Methods for Preparation of Mannich Bases, Formazan, Normal and Cyclic Sulfur Compounds”, 1st edition Evince pub Publishing House;2018, ISBN: 978-93-87905-19-1.
- Nagham Mahmood Aljamali., “Alternative Methods in Organic Synthesis”.,1th–Edition, Eliva Press SRL, 2020 ., ISBN: 9798680201176.
- Matheus ME, de Almeida Violante F, Garden SJ. Isatins inhibit cyclooxygenase-2 and inducible nitric oxide synthase in a mouse macrophage cell line. Eur J Pharmacol. 2007; 556 :200–6.
CrossRef - Perro, S. Reculusa, E. Bourgeat-Lami, E. Duguet, S. Ravaine. Synthesis of hybrid colloidal particles: from snowman-like to raspberry-like morphologies Colloids Surf, Physicochem. Eng. Asp., 284 (2006), pp. 78-83.
CrossRef - Pera-Titus, L. Leclercq, J.-M. Clacens, F. De Campo, V. Nardello-RatajPickering interfacial catalysis for biphasic systems: from emulsion design to green reactions., Angew Chem. Int. Ed., 54 (7) (2015), pp. 2006-2021.
CrossRef - Nagham Mahmood Aljamali, “Spectral and Laboratory Diagnostics of Compounds”., 1th –Edition, 2021, Eliva Press SRL., ISBN: 9781636482118.
- Nagham Mahmood Aljamali, Imad Kareem Alwan Alsabri., Development of Trimethoprim Drug and Innovation of Sulfazane-Trimethoprim Derivatives as Anticancer Agents ., Biomedical & Pharmacology Journal, March 2020., Vol. 13, (2), p. 613-625 ., http://dx.doi.org/10.13005/bpj/1925 .
CrossRef - Imad Kareem Alwan Alsabri, Hasaneen Kudhair Abdullabass ,Nagham Mahmood Aljamali .,Invention of (Gluta.Sulfazane-Cefixime) Compounds as Inhibitors of Cancerous Tumors., Journal of Cardiovascular Disease Research, 2020,11, 2., 44-55 ., DOI: 10.31838/jcdr.2020.11.02.09 .
CrossRef - Hasaneen Kudhair Abdullabass, Aseel Mahmood Jawad ,Nagham Mahmood Aljamali . Synthesis of drugs derivatives as inhibitors of cancerous cells., Biochem. Cell. Arch, 20 (2) – October 2020.
- Casagrande, P. Fabre, E. Raphael, M. VeyssieJanus beads: realization and behavior at water oil interfaces., Europhys. Lett., 9 (3) (1989), pp. 251-255.
CrossRef - -J. Zhou, L. Fang, Z. Fan, B. Albela, L. Bonneviot, F. De Campo. .Tunable catalysts for solvent-free biphasic systems: pickering interfacial catalysts over amphiphilic silica nanoparticles., J. Am. Chem. Soc., 136 (13) (2014), pp. 4869-4872.
CrossRef - Mehta SL, Manhas N, Raghubiz R. Molecular targets in cerebral ischemia for developing novel therapeutics . Brain Res Rev. 2007;54:34–66.
CrossRef - Meaaed M, Nagham Mahmood Aljamali, Nadheema A A., “Preparation, Spectral Investigation, Thermal Analysis, Biochemical Studying of New (Oxadiazole -Five Membered Ring)-Ligands”., Journal of Global Pharmacy Technology, 2018;10,1,20-29.
- Liu, J. Hu, X. Yu, X. Xu, Y. Gao, H. Li. Preparation of Janus-type catalysts and their catalytic performance at emulsion interface., J. Colloid Interface Sci., 490 (2017), pp. 357-3564.
CrossRef - Nagham Mahmood Aljamali. Survey on Methods of Preparation and Cyclization of Heterocycles. International Journal of Chemical and Molecular Engineering. 2020; 6(2): 19–36p.
- W. Seh, S. Liu, S.-Y. Zhang, M.S. Bharathi, H. Ramanarayan, M. Low. Anisotropic growth of titania onto various gold nanostructures: synthesis, theoretical understanding, and optimization for catalysis., Angew Chem. Int. Ed., 50 (43) (2011), pp. 10140-10143.
CrossRef - Mieaad M, Nagham Mahmood Aljamali, Wassan Ala Shubber., Sabreen Ali Abdalrahman .”New Azomethine- Azo Heterocyclic Ligands Via Cyclization of Ester”., Research Journal of Pharmacy and Technology, 2018, 11,6, 2555-2560 ., DOI : 5958/0974-360X. 2018. 00472.9 .
CrossRef - A. Ismail, D.W. Bahnemann, I. Bannat, M. Wark Gold nanoparticles on mesoporous interparticle networks of titanium dioxide nanocrystals for enhanced photonic efficiencies ,J. Phys. Chem. C., 113 (17) (2009), pp. 7429-7435.
CrossRef - Shafiq, Iqrash; Shafique, Sumeer; Akhter, Parveen; Yang, Wenshu; Hussain, Murid . (2020) . “Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: A technical review”. Catalysis Reviews. 0: 1–86.
CrossRef - Nagham Mahmood Aljamali, Intisar Obaid Alfatlawi. “Synthesis of Sulfur Heterocyclic Compounds and Study of Expected Biological Activity”, Research J. Pharm. and Tech., 2015, 8,9 ,1225-1242 , DOI: 10.5958/0974-360X.2015 .00224.3.
CrossRef - Wei, Hui; Wang, Erkang (2013). “Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes”. Chemical Society Reviews. 42 (14): 6060–93. doi:1039/C3CS35486E. ISSN 1460-4744. PMID 23740388.
CrossRef - Intisar Obaid Alfatlawi, Nuha S S, Zainab M J ,Nagham Mahmood Aljamali, Intisar O A. “Synthesis of New Organic Compounds Via Three Components Reaction with Studying of (Identification, Thermal Behavior, Bioactivity on Bacteria of Teeth)”., Journal of Global Pharma Technology. 2017;11,9, 157-164.
- Naumann d’Alnoncourt, Raoul; Csepei, Lénárd-István; Hävecker, Michael; Girgsdies, Frank; Schuster, Manfred E.; Schlögl, Robert; Trunschke, Annette (2014). “The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts”. Journal of Catalysis. 311: 369–385.
CrossRef - Mokrani, Touhami; van Reenen, Albert; Amer, Ismael (2015).”Molecular weight and tacticity effect on morphological and mechanical properties of Ziegler–Natta catalyzed isotactic polypropylenes”. Polímeros. 25 (6): 556–563. doi:1590/0104-1428.2158 . ISSN 0104-1428.
CrossRef - Shireen R. Rasool, Nagham Mahmood Aljamali ,Ali Jassim Al-Zuhairi., Guanine substituted heterocyclic derivatives as bioactive compounds., Biochem. Cell. Arch. Vol. 20, Supplement 2, pp. 3651-3655, 2020 ., DocID: https://connectjournals.com/03896.2020.20.3651.
CrossRef - Dub, Pavel A.; Gordon, John C. (2018). “The role of the metal-bound N–H functionality in Noyori-type molecular catalysts”. Nature Reviews Chemistry. 2 (12): 396–408.
CrossRef - Aseel Mahmood Jawad., Nagham Mahmood Aljamali, Saher Mahmood Jawd., Development and Preparation of ciprofloxacin Drug Derivatives for Treatment of Microbial Contamination in Hospitals and Environment, Indian Journal of Forensic Medicine & Toxicology, 2020 ,14, 2, p:1115-1122.
- S FJawad, Nagham Mahmood Aljamali , Preparation, Investigation and Study of Biological Applications of Tyrosine Derivatives against Breast Cancer Cells ., NeuroQuantology ,September 2021 ,Volume 19 , Issue 9 , Page 117-125 .,doi: 10.14704/nq.2021.19.9.NQ21144 .
CrossRef - Clark, Jim (2013). “Types of catalysis”. Chemguide. Bård Lindström and Lars J. Petterson (2003) “A brief history of catalysis” Cattech, 7 (4) : 130–38.
CrossRef - Pisarewicz K, Mora D, Pflueger F, Fields G, Marí F (2005). “Polypeptide chains containing D-gamma-hydroxyvaline”. J Am Chem Soc. 127 (17): 6207–15
CrossRef








