Manuscript accepted on :February 28, 2016
Published online on: 29-03-2016
Plagiarism Check: Yes
Mohammadreza Shahmohammadi1, Reza Jalil Khoshnod1 , Nima Mohseni Kabir1 and Peyman Karimi Goudarzi2*
Department of Neurosurgery , Shahid Beheshti University of Medical Sciences , Tehran , Iran Department of Neurosurgery , AJA University of Medical Sciences , Tehran , Iran Corresponding Author Email: pedram_kg@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/905
Abstract
Multiple Sclerosis (MS) is a chronic disease with an unknown cause that results in inflammation and destruction of Myelin in central nervous system (CNS). The final result is disruption of nervous system functions. This disorder commences at the age of 10 to 60 and her frequency among women is two to three times more than what is recorded among men. As we move further from the equator, the prevalence of this disease increases, so does its variation in various races. The commonest type of MS is the Relapsing-Remmiting multiple Sclerosis. Oncostatin M (OSM) is a Cytokine in Interleukin 6 family (IL-6). OSM is secreted in patients with multiple Sclerosis. OSM improves E2 expression of prostaglandin and cyclooxygenases-2 in astrocytes. This action is of great significance in the efficiency of CNS endothelial cells and creation of blood brain barrier (BBB). The present study seeks to study the frequency distribution of Oncostatin M in the patients suffering from Relapsing-Remmiting multiple Sclerosis and healthy control individuals. The present research has been conducted using a case-control method. 83 patients who had resorted to a private hospital in Tehran due to having the clinical symptoms of multiple Sclerosis whose disease was confirmed by the neurologists based on Mc Donald criteria were randomly selected for this research. All the above-mentioned patients entered the study on the condition of meeting the acceptance criteria. The population was divided into two groups, namely case (patients with MS) and control (healthy people without chronic inflammatory or neurogenetic disease). 5 cc blood was obtained on anticoagulant EDTA and preserved in a temperature of -80 for the later phases of the research. Finally, ELISA test was conducted utilizing EastBiopharm Company protocols and the data resulting from the analysis of micro-plate reader device was collected for statistical analysis phase. The raw data was then entered in SPSS version 19 and independent t-test and Spearman Correlation tests were used to analyze the data. The significant level of the possible value was set to (P-Value < 0.05). Studying the serum level of OSM in both groups (patients and healthy individuals) is indicative of the fact that although the OSM serum density in patients (634.68 658.43(pg/ml)) was much less than the level observed in healthy cases (860.57 891/16(pg/ml)), this difference was by no means statistically significant (P-Value > 0.05). The results of studying the correlation between the expanded disability status scale (EDSS) and the Oncostatin M density level in the group of the patients indicated no statistically significant relationship between the parameters in the group mentioned (P-Value = 0.471). MS plays no major role in changing OSM serum level and EDSS is a variable independent from OSM serum density parameter.
Keywords
Multiple Sclerosis; CNS; RRMS; OSM; Oncostatin M
Download this article as:| Copy the following to cite this article: Shahmohammadi M, Khoshnod R. J, Kabir N. M, Goudarzi P. K. Frequency of OSM in Patients With Relapsing-Remmiting Multiple Sclerosis . Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Shahmohammadi M, Khoshnod R. J, Kabir N. M, Goudarzi P. K. Frequency of OSM in Patients With Relapsing-Remmiting Multiple Sclerosis. Biomed Pharmacol J 2015;9(1). Available from: http://biomedpharmajournal.org/?p=6651 |
Introduction
Multiple Sclerosis (MS) is a chronic, inflammatory and nervous autoimmune disease which influences central nervous system (CNS). MS attacks axons with Myelin in the central nervous system and this attack damages Myelin and axons. The commonest type of MS observed in 85% of those afflicted is the Relapsing-Remmiting multiple Sclerosis. RMMS is characterized with the sudden intensification of the symptoms and the subsequent reduction of those symptoms (1). It can be stated that temporary attacks to Myelin and axons by immune cells will contribute to the symptoms of this disease (2). The symptoms of this disease begin to show themselves between the age of 20 to 40 years old, and this disorder constitutes the major cause of non-traumatic disability in young adults. The initial symptoms rarely emerge before the age of 10 or after the age of 60. The studies conducted in this area have shown that women are twice as susceptible to this disease as men, except for the progressive-primitive (PP) type where gender shows no privilege in getting the disease (3). 2.5 million people are suffering from MS in the world 400 thousand of whom live in the US. During the period of April 2003 to July 2010, the prevalence of MS in Tehran has been 73.3 in each 100 thousand people. Based on the statistics of 2010 to 2011, the number of new cases of MS identified by Tehran Society of MS was 443 people. Compared to 43.8 in 2007, it is seen that her epidemics has risen significantly. Further to the previous reports, Tehran is one of the areas with high factor risk of MS in Asia and Oceania (4). Causes of MS have not been identified yet, however, it is possible that a mixture of genetic susceptibility and a non-genetic trigger such as a virus (EBV has been identified as the most important virus candidate for MS) (5), metabolism and environmental factors are involved in forming an autoimmune, self-reliant and relapsing disorder (1). Despite the uncertainty about the etiology of MS, there are various evidences which suggest a role for pathogen T and B lymphocytes in development and progress of the disease. These cells, also known as self-attacking cells, find their path to central nervous system (CNS) and disrupt the functioning of cells (6). The cells providing APCs antigen produce antigens associated with the activation of some types of TH cells known as TH-17 and TH-1 self-attacking and pre-inflammatory cells. B cells and Monocytes are also activated. Self-attacking T cells get connected to the sticky molecules of the endothelial surface of CNS capillaries and, with the aid of Protease and Chemokines, pass through the blood-brain barrier. In CNS, target antigens (myelin basic protein, myelin-associated glycoprotein, myelin-Oligodendrocyte glycoprotein, proteolipid protein, aB-crystallin, phosphodiesterases) are identified and the activated open T cells intensify the immune response. The production of pre-inflammatory Th cells increases significantly, B cells will turn into plasmocyte cells producing mature antibody and Monocytes turn into active Macrophages. The whole set of these immune cells produces Cytokines, protease, free radicals, nitrite oxide, glutamate and other anxiety factors which will destroy the Myelin and oligodendrocytes. Depending on the location and intensity of damage, destruction of Myelin results in weakening of neural messages or even prevents its transfer. It will finally contribute to the emergence of the neurological symptoms of MS (3). Various studies are indicative of the fact that Oncostatin M (OSM) is a Cytokine from interleukin 6 family (7) and it plays a major role in cellular processes such as white globules attraction, formation of T cells outside thymus, immune tune up of brain’s endothelial cells, deterioration of the nervous tissue and higher synthesis of P-selections (8, 9). What’s more, OSM improves E2 expression of prostaglandin and cyclooxygenases-2 in astrocytes. This action is of great significance in efficiency of CNS endothelial cells and creation of blood brain barrier (BBB). E2 prostaglandin or PGE2 is the most frequent group of prostaglandin lipids and plays a major role in inflammation and stress of tissues. Precursors of prostaglandins are synthesized through the effect of A2 Phospholipase on membrane phospholipids, provision of the necessary Arachidonic acid, and the influence of Cyclooxygenase. OSM utilizes both types of receptors to discharge messages. These receptors include gp130 and OSMRß Heterodimers. Utilizing antibodies that nullify these receptors will cause a major reduction in PGE2 (10). Keeping in mind the important roles of OSM such as inflammatory processes (attraction of white globules, participating in TH-1 and TH-17 responses and Leukocyte adhesion), formation of BBB, participation in recovery of Demyelinated plaques and lack of an extensive and focused study on OSM structure and its detailed role in MS Pathogenesis, its major evaluation in plasma of the patients and healthy treatments can be a positive first step in proving the theory of OSM interference in MS mechanism in the population of the patients in Tehran and the whole Iran. taking into consideration the discussions put forward in this research, the researchers set to conduct a study titled frequency distribution of Oncostatin M in the patients suffering from Relapsing-Remmiting multiple Sclerosis.
Material and methodology
A case-control approach was taken to conduct this study. The present study was conducted on patients resorting to a private hospital in Tehran in 2015 due to exhibiting the clinical symptoms of multiple Sclerosis. The entrance criteria for this study included factors such as having been diagnosed with RRMS disease based on Mc Donald criteria and MRI by the neurologist, patients’ cooperation to donate blood after gaining their consent, RRMS patients with at least two complete or relative relapsing and remmiting, an EDSS degree ranging from 0 to 5, and no affliction with other diseases of central nervous system or other special or chronic disease. Patients who did not cooperate for blood donation or were suffering from other inflammatory, autoimmune and infectious diseases or MS groups such as PPMS and SPMS were discarded. The criteria for participation of healthy people in the study included factors such as cooperation for blood donation after gaining their consent, being matched with the patients in terms of age, gender and race. The individuals took part in the research voluntarily after their consent had been gained in accordance with medical ethics fundamentals. People in this study were divided into the case and witness groups. 60 non-relative patients whose disease was confirmed by neurologists through MRI and McDonald Protocol were selected in accordance with the medical ethics committee protocols. Some 23 healthy people without any past records of autoimmune and inflammatory diseases who had come for blood donation and matched the patients in terms of age, gender and race were selected randomly to take part in the study). 5 cc blood was obtained on anticoagulant EDTA and stored in a temperature of -80 for the future phases of the research. Finally, ELISA test was conducted utilizing East Biopharm Company protocols and the data resulting from the analysis of micro-plate reader device was relegated to the statistical analysis phase. The samples utilized for this kit included human serum and plasma and other relevant tissue liquids. This kit measures the density of human OSM through ELISA sandwich double antibody method. Based on the commercial kit protocol, the standard determined quantities along with the samples and the antibody conjugated with enzyme were added to micro-plate cap and exposed to a temperature of 37 for 60 minutes after preparation of solutions, samples and standards. Then, the micro-plates were rinsed 5 times and A and B Chromogens were added to them and the enzyme reaction was conducted in a temperature of 37 for 10 minutes. Having added the stopper solution, the optical density (OD) of the color produced was measured by micro-plate reader device and the results were calculated in comparison with the standard. The raw data obtained was entered to SPSS version 19 software. Then, K-S test was utilized to study the normality of data for all variables. Appropriate statistical tests were utilized based on the normality status. The Independent t-Test was used as a parametric statistical test, while Spearman Correlation was utilized as a non-parametric test. The statistical significance limit in this study (P-value) was set to a level less than 0.05.
Results
First the variables introduced and then the raw data were studied using appropriate statistical tests. The present study was conducted on 83 patients resorting to a private hospital in Tehran. 53 participants (63.9%) were female and the remaining 30 (36.1%) were male. People were divided into case and control groups. 81.7% of the participants in the case group were women and men constituted the remaining 18.3%. The control group was composed of 17.4% women and 82.6% men. The age of the people in the case group ranged from 15 to 49 with an average age of 30.507.87, while the ages of people in the control group were in the range of 20 to 51 years old with the average age of 31.267.19. The initial symptoms in this disease are divided into 12 groups (cord/one-sided spasms, right leg numbness, right side numbness, blurred vision, hand numbness, looking daggers, right hand numbness, feeling dizzy, headache, feet numbness, left body side numbness and chest numbness). The highest frequency in the case group is associated with blurred vision with 13 (21.7) reported cases, while the lowest frequency corresponds with cord/one-sided spasms, hand numbness, right hand numbness and feet numbness each with only 1 (1.2) case reported. The most frequent blood groups in the Case and Control groups were O+ (23.3%) and A+ (34.8%) respectively. The least frequent blood groups in the Case group was B– (1.7%), while blood groups A– and B– (4.3% each) had the least frequencies among members of the Control group. The number of the attacks over the last 2 years was studied in all members of the Case group. According to results, 11 people experienced such attacks only once, 4 experienced them twice, and only 1 individual experienced the attacks 4 times in the course of the last 2 years.
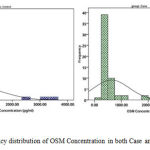 |
Figure 1: Frequency distribution of OSM Concentration in both Case and Control groups
|
Utilizing Independent t-Test statistical test, the relationship between OSM Concentration and MS disease was studied. The mean of this variable in both groups studied (patients with RRMS and the witness group) was taken into consideration. The results of this test summarized in table 1 show that the serum level of OSM in the group of patients (634.68658.43(pg/ml)) is lower than the serum level of OSM in healthy people (860.57891.16(pg/ml)), but this difference can’t be significant in terms of the statistical tests (P-Value > 0.05).
Table 1: Distribution and evaluation of OSM Concentration correlation in various groups
| group | OSM concentration | MenS.D (pg/ml) | Min (pg/ml) | Max (pg/ml) | P-Value | Df |
| Case | 60 | 634.68658.43 | 245.29 | 3154.10 | 0.210 | 81 |
| Control | 23 | 860.57891.16 | 336.88 | 3382.04 |
We also studied the relationship between EDSS and OSM Concentration in the group of patients (Figure 2). It is necessary to mention that EDSS is a scale to determine the disability in patients suffering from MS. The results of statistical analysis using Spearman’s Correlation and Correlation Coefficient test show no significant statistical relationship between the two parameters in the above-mentioned group (P-Value = 0.471, Spearman coefficient = 0.142).
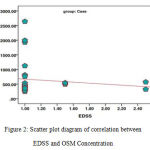 |
Figure 2: Scatter plot diagram of correlation between EDSS and OSM Concentration
|
Discussion
MS (multiple Sclerosis) is a chronic inflammatory disease which attacks the central nervous system (CNS). It is one of the most important causes of disability among the youth. There is no certain treatment for this disease and the medicines taken by the patients merely provide auxiliary and controlling treatments (11). This disease is 2 to 3 times as common among women as among men. MS is seen in various forms and types. RRMS is the most common type of it. The symptoms of this disease are indicative of the fact that attacks in the patient intensify and stop and new symptoms are observed in the patient after each attack or the former symptoms intensify (12). There are various clinical symptoms for this disease. The most common symptoms are feeling dizzy, double vision, Cranial nerve palsies, eye movement disorder, and sensory or motor symptoms. Signaling studies conducted over previous years are indicative of the fact that OSM plays a major role in the mechanism of MS. OSM is a Cytokines from the members of interleukin 6 (IL-6) family (13) secreted in patients suffering from MS by activated glial cells, macrophage, T lymphocytes, Monicyte and dendrite cells (14). Based on the findings of the scientist, Oncostatin M shortens the death process of neurons due to too much induction of neurotransmitters (Excitotoxicity) in Invitro and Invivo environments (16, 15) and prevents the death of membrane neurons (17). OSM can reduce the size of Myelitis and induce the nervous recovery and treatment of the neurons’ performance (17). Although various researches have been conducted on OSM and while its major role in protecting neurons has been made clear, some researchers confirm the protective role of OSM during the Demyelination of neurons and name it as an appropriate candidate to reduce CNS damages in Demyelinating diseases such as MS (18). Some other functional features of OSM such as her role in the process of demylunation of neurons have still remained unclear. OSM may increase the expression of interleukin and induce polarization of Myeloid cells towards anti-inflammatory M2 Phenotype in Invivo environment. In 2002, Ensoli studied the serum density of TNF, TNF, TNF and Oncostatin M in MS patients. As the results indicate, OSM serum level on the average was higher than what was observed in healthy people, but there was no statistically significant difference between them. However, the serum level of TNF, TNF, TNF in the patients was below the detection limit (9). Although the present research found that the serum density of OSM among the patients was much less than what was observed in healthy people, the fact that this difference was statistically insignificant was in line with findings of Ensoli et al. Researchers have conducted various studies to show that secretion of OSM in patients with respiratory tract illnesses (such as Allergic rhinitis, Asthma, and IPF (19) and lung disease) is much more than healthy patients (22, 21). OSM serum level can also increase the neuro-cognitive disorders with AIDS (HAND) (24, 23). In 2012, Linag et al. showed that OSM serum level in liver patients with cancer marker GPV3 is 6 to 7 times as strong as healthy people (25). We may draw the following final conclusion: although the OSM serum density in patients is much less than healthy people, no significant difference was observed between them. Keeping in mind the results of the previous studies, one may conclude MS has no significant influence on OSM production. OSM density and EDSS are two independent variables between which there is no correlation.
References
- Goldenberg MM. Multiple Sclerosis review. Pharmacy and Therapeutics. 2012;37(3):175.
- Christensen JR, Börnsen L, Hesse D, Krakauer M, Sørensen PS, Søndergaard HB, et al. Cellular sources of dysregulated cytokines in relapsing-remitting multiple Sclerosis. Journal of neuroinflammation. 2012;9(1):1-12.
- Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple Sclerosis. Am J Manag Care. 2013;19(2 Suppl):S15-20.
- Etemadifar M, Maghzi A-H. Sharp increase in the incidence and prevalence of multiple Sclerosis in Tehran, Iran. Multiple Sclerosis Journal. 2011;17(8):1022-7.
- Maghzi A-H, Marta M, Bosca I, Etemadifar M, Dobson R, Maggiore C, et al. Viral pathophysiology of multiple Sclerosis: A role for Epstein-Barr virus infection? Pathophysiology. 2011;18(1):13-20.
- Gabibov AG, Belogurov AA, Lomakin YA, Zakharova MY, Avakyan ME, Dubrovskaya VV, et al. Combinatorial antibody library from multiple Sclerosis patients reveals antibodies that cross-react with myelin basic protein and EBV antigen. The FASEB Journal. 2011;25(12):4211-21.
- Kerfoot SM, Raharjo E, Ho M, Kaur J, Serirom S, McCafferty D-M, et al. Exclusive neutrophil recruitment with Oncostatin M in a human system. The American journal of pathology. 2001;159(4):1531-9.
- Modur V, Feldhaus M, Weyrich A, Jicha D, Prescott S, Zimmerman G, et al. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. Journal of Clinical Investigation. 1997;100(1):158.
- Ensoli F, Fiorelli V, Lugaresi A, Farina D, DeCristofaro M, Collacchi B, et al. Lymphomononuclear cells from multiple Sclerosis patients spontaneously produce high levels of Oncostatin M, tumor necrosis factors α and β, and interferon γ. Multiple Sclerosis. 2002;8(4):284-8.
- Repovic P, Mi K, Benveniste EN. Oncostatin M enhances the expression of prostaglandin E2 and cyclooxygenase‐2 in astrocytes: Synergy with interleukin‐1β, tumor necrosis factor‐α, and bacterial lipopolysaccharide. Glia. 2003;42(4):433-46.
- Alehashmi A. Evaluation of cognitive disorders in MS patients in Ghaem Hospital. Mashhad University of Medical Sciences.1382.
- Wood AJ, Rudick RA, Cohen JA, Weinstock-Guttman B, Kinkel RP, Ransohoff RM. Management of multiple Sclerosis. New England Journal of Medicine. 1997;337(22):1604-11.
- Tanaka M, Miyahima A. Onconstatin M, a multifunctional cytokine. Reviews of physiology, biochemistry and pharmacology: Springer; 2004. p. 39-52.
- Ruprecht K, Kuhlmann T, Seif F, Hummel V, Kruse N, Brück W, et al. Effects of Oncostatin M on human cerebral endothelial cells and expression in inflammatory brain lesions. Journal of Neuropathology & Experimental Neurology. 2001;60(11):1087-98.
- Weiss TW, Samson AL, Niego Be, Daniel PB, Medcalf RL. Oncostatin M is a neuroprotective cytokine that inhibits excitotoxic injury in vitro and in vivo. The FASEB Journal. 2006;20(13):2369-71.
- Moidunny S, Dias RB, Wesseling E, Sekino Y, Boddeke HW, Sebastião AM, et al. Interleukin‐6‐type cytokines in neuroprotection and neuromodulation: Oncostatin M, but not leukemia inhibitory factor, requires neuronal adenosine A1 receptor function. Journal of neurochemistry. 2010;114(6):1667-77.
- Durrani N, Koehn P, editors. Improving machine translation via triangulation and transliteration. Proceedings of the 17th Annual Conference of the European Association for Machine Translation (EAMT), Dubrovnik, Croatia; 2014.
- Janssens K, Maheshwari A, Van den Haute C, Baekelandt V, Stinissen P, Hendriks JJ, et al. Oncostatin M protects against demyelination by inducing a protective microglial phenotype. Glia. 2015.
- Mozaffarian A, Brewer AW, Trueblood ES, Luzina IG, Todd NW, Atamas SP, et al. Mechanisms of Oncostatin M-induced pulmonary inflammation and fibrosis. The Journal of Immunology. 2008;181(10):7243-53.
- Luzina IG, Atamas SP, Wise R, Wigley FM, Choi J, Xiao HQ, et al. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis & Rheumatism. 2003;48(8):2262-74.
- Simpson JL, Baines KJ, Boyle MJ, Scott RJ, Gibson PG. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Experimental lung research. 2009;35(9):781-94.
- Kang HJ, Kang JS, Lee SH, Hwang SJ, Chae SW, Woo JS, et al. Upregulation of Oncostatin M in allergic rhinitis. The Laryngoscope. 2005;115(12):2213-6.
- Vecchiet J, Dalessandro M, Falasca K, Travasi F, Zingariello P, Schiavone C, et al. Increased production of Oncostatin-M by lymphomononuclear cells from HIV-1-infected patients with neuroAIDS. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2003;32(4):464-5.
- Repovic P, Benveniste EN. Prostaglandin E2 is a novel inducer of Oncostatin-M expression in macrophages and microglia. The Journal of neuroscience. 2002;22(13):5334-43.
- Liang H, Block TM, Wang M, Nefsky B, Long R, Hafner J, et al. Interleukin-6 and Oncostatin M are elevated in liver disease in conjunction with candidate hepatocellular carcinoma biomarker GP73. Cancer Biomark. 2012;11(4):161-71. Epub 2012/11/13.







