Manuscript accepted on :May 15, 2014
Published online on: 21-12-2015
Plagiarism Check: Yes
Ali Yadollahpour and Zohre Rezaee
Department of Medical Physics, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
DOI : https://dx.doi.org/10.13005/bpj/452
Abstract
Electroporation is a highly effective method to increase permeability of cell membrane by using series of short intense electric pulses. Using this technique, we can introduce small and large molecules into cells. The electroporation of biological membranes has various applications in molecular biology and medicine. Despite the numerous applications of electroporation, the detailed effects of electric pulse on biological membranes as well as exact mechanisms of pore formation in living cells are not well understood. Several in-vitro and in-vivo experimental studies have been conducted to determine the mechanisms of action of electroporation in various types of membranes. Because of the small spatial and fast temporal scales of this process, direct observation of electroporation is difficult, theoretical models and molecular dynamic simulations have been developed to facilitate the interpretation of experimental data and the understanding of the mechanisms of action of electroporation. The role of phospholipids in respond to external electric fields, behavior of water dipoles in the complex electric field landscape of the membrane interface and reorganization of water dipoles in pore formation process have been proposed in these studies. Pore characteristics such as life time, ion selectivity, size, kinetics of formation as well as number of pores are significant factors in the proposed mechanism of action. The present study reviews the different mechanisms of action of electroporation proposed by different experimental and modeling studies.
Keywords
Electroporation; Mechanisms of action; cell membrane; membrane pore
Download this article as:| Copy the following to cite this article: Yadollahpour A, Rezaee Z. Electroporation as a New Cancer Treatment Technique: A Review on the Mechanisms of Action. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Yadollahpour A, Rezaee Z. Electroporation as a New Cancer Treatment Technique: A Review on the Mechanisms of Action. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2841 |
Introduction
The membrane of a living cell is about 5 nm thick, and primarily made up of a phospholipid bilayer molecule. A cytoskeletal network gives structural reinforcement, and embedded proteins carry out the necessary exchange of material between the inside and outside of the cell. The polar heads of the phospholipid molecules stick out into the aqueous intracellular and extracellular solution. In the middle of the bilayer the apolar tails, with a length of 16–18 carbons, are directed towards each other[1]. This cell membrane acts as a barrier that hinders the free diffusion of ions and molecules through cell membrane separating cytoplasm and external medium. However, the permeability of membrane can be transiently increased when a cell is exposed to the short, intense, external electric pulses [2-5]. Stampfli was the first to suggest the reversible breakdown of cell membranes in response to external electric fields[6]. A few years later Coster observed the phenomenon of a “punch through” of the cell membrane by an electric field[7]. Discussions on the increase in permeability of the plasma membrane of a cell due to the formation of pores, an effect subsequently named “electroporation” by Neumann and Rosenheck, appeared in 1972[8]. The effectiveness of this phenomenon depends on several parameters that the influence of extracellular media, pulse shape, field strength, pulse duration, time window and resting transmembrane voltage on cell electropermeabilization was investigated in many studies[9-16]. As of today, the medical applications of electroporation include gene electrotransfer[17], transdermal drug delivery, tumor and tissue ablation, and the electrochemotherapy of tumors[18, 19]. After the first description of in vitro gene transfer to living cells by electroporation (EP) this technique has been widely used. Innovation and sophistication have taken EP into a second era, where pulses well controlled with regard to both amplitude and duration can be applied, also in the in vivo setting. Gene delivery by EP has proven efficient in various tissues including tumor, liver, skin and muscle[20]. The use of electroporation to transport non-permeant chemotherapy drugs has been termed electrchemotherapy. Extensive invitro or invivo study demonstrated that electrochemotherapy is efficient, safe, inexpensive and local treatment without significant side effects that can be used for various type of superficial tumors[21-32]. Namely, electrochemotherapy was, until 2010 only used for superficial and accessible tumor nodules, with an approximately 80% objective response rate[33, 34] but the first study of deep-seated tumor electrochemotherapy based on numerical treatment planning was reported in 2010. In this, the first reported clinical case, deep-seated melanoma metastasis in the thigh of the patient was treated by electrochemotherapy, according to a treatment plan obtained by numerical modeling. the presented work demonstrates that treatment of deep-seated tumor nodules by electrochemotherapy is feasible and sets the ground for numerical treatment planning-based electrochemotherapy [35].In contrast to reversible EP used in ECT and EGT, the irreversible electroporation that was first introduced in 2005[36] leads to cell death and can be used in various applications, including tumor ablation[37, 38]. this technique is based upon the application of strong electrical fields rather than the deposition of heat or chemical agents[39, 40]. Neal et al demonstrated that human breast cancer tumors orthotopically implanted in the mouse can be successfully treated using IRE. And IRE could be an advantageous alternative to surgical resection for breast conserving therapy[19]. The recent studies of in vivo electropermeabilization indicate a shift towards the combining this phenomenon with other techniques such as sonoporation[41, 42] and immunotherapy[43]. Future studies will be to test this multimodal poration strategy for delivery of larger genetic molecule such as siRNA or even DNA-plasmid into mammalian cells.
Experimental or theoretical models have been proposed to explain the mechanism of this reversible membrane electropermeabilization and its potentiality to allow the access of non-permeant molecules inside the cells, Nevertheless, the molecular definition of the “Transient Permeable Structures” is not yet known. The purpose of this paper is to review different studies about mechanism of electroporation.
Theory of Electroporation
The transmembrane potential induced in a cell by an external field is generally described by the Eq.1:
![]()
Where Vm is the trans-membrane potential, a form factor describing the impact of the cell on the extracellular field distribution, the applied electric field the cell radius, and is the polar angle with respect to the external field. The value for the factor is listed by many authors as 1.5; however, this factor is dependent on a number of different factors[14]. Electroporation is achieved when the Vm superimposed on the resting transmembrane potential is larger than a threshold, Vm is generally reported to be in the order of 1 V [44], although an experimental and theoretical study has later described it as being 200 [45]. This voltage affects the functioning of voltage-gated membrane channels, initiates the action potentials, it can lead to cell membrane electroporation[46]. Permeabilization will initially happen at the pole of the cell facing the positive electrode, because owing to the negative interior of the cell this is where the capacitance of the membrane is first exceeded when an external field is applied(fig1)[47]. Despite the wide laboratory use of electroporation, the details of the effects of electric fields on biological membranes, and particularly the exact molecular mechanisms of pore creation in living cells, are not well understood[48]. Numerous experimental and theoretical studies have been aimed at revealing the mechanism of electroporation.
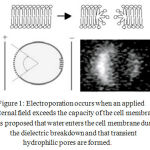 |
Figure 1: Electroporation occurs when an applied external field exceeds the capacity of the cell membrane. It is proposed that water enters the cell membrane during the dielectric break down and that transient hydrophilic pores are formed.
|
Experimental Studies
Several experimental studies have been carried out to determine the mechanism of electroporation in various types of membranes ranging from artificial lipid bilayers to chick myocyte monolayers [49-57]. These studies investigated the properties of pore formation and resealing using pulse charge techniques [58, 59] measured the kinetics of electroporation in voltage-clamped membranes[60], tracked the movement of ions and fluorescent dyes across electroporated membranes[61], imaged the transmembrane potential using voltage-sensitive dyes[62] and visualized large pores using freeze-fracture electron microscopy[52]. Beside of these constant current method can be applied for continuous observation of pore dynamical behavior[63]. Litster[64]and Taupin, Dvolaitzky, and Sauterey[65] were probably the first to mention the role of thermal fluctuations in pore formation, and the existence of a threshold pore formation energy. Also under electrical stress, there are two types of membrane behaviors: irreversible and reversible electrical breakdown. In the case of irreversible electrical breakdown a measurable increase in membrane conductance rapidly leads to mechanical rupture of the membrane[66, 67]. But by using short-intense electric pulses (reversible) even after five to six orders of magnitude increase of conductance, membrane conductance quickly drops to the initial level upon voltage decrease[51, 58].
To reveal the electroporation process in the early stage, conductance variations associated with the formation of transient metastable single pores in unmodified bilayer lipid membrane were studied under high electric fields [68]. In addition, single metastable nanopores are formed before the actual electroporation under constant-current conditions [69]. This finding showed that transmembrane potential value is more relevant in triggering electroporation process than the current value. However, the current value defines the speed of membrane charging and transitions pace between subsequent stages of electroporation. Also, this study showed that the planar membrane is significantly more susceptible to electroporation when its capacitance is low, which is probably related to its thickness and structure.
Direct observation of electroporation is difficult because of the small spatial and fast temporal scales of this process. Therefore, theoretical models have been developed to facilitate the interpretation of experimental data and the understanding of the mechanism of electroporation.
Theoretical Models
A number of theoretical models have been proposed for the explanation of electroporation. Hydrodynamic, elastic, hydroelastic, viscohydroelastic, phase transition, domain-interface breakdown model and aqueous pore formation are examples of these models.The hydrodynamic, the elastic, the viscoelastic, and the viscohydroelastic models consider electroporation as a large scale phenomenon, with no direct role attributed to the molecular structure of the membrane. The phase transition model and the domain-interface breakdown models represent the other extreme, attempting to explain the electroporation by the properties of individual lipid molecules and the interactions between them. The aqueous pore formation offers a compromise between these two approaches considering the electropermeabilization as a result of the formation of transient aqueous pores in the lipid bilayer. Each pore is formed by a large number of lipid molecules, while the shape, size, and stability of the pore are strongly influenced by the nature of these molecules and their local electrochemical interactions[70].
An applied electric field is capable of affecting the hydrated polar head groups of lipids, which leads to the formation of hydrophilic pores. The field-induced translational motions of the polar lipids in the curved pore wall also rationalize the huge acceleration of lipid flip-flop and the eventual redistribution of the lipids and charges from the internal membrane monolayer to the outer monolayer and vice versa[71]. One deficiency of the model is the lack of consideration of the hydration of proteins and lipid polar headgroups as well as the hydrated ions that are subject of transport through the pores[70]. It should be taken into account that the lipids adjacent to the aqueous molecules inside of the pore are reoriented in a manner that their hydrophilic heads are facing the pore, while their hydrophobic tails are hidden inside the membrane[72].
Today, the aqueous pore formation model is considered to be the most convincing explanation of electroporation. To improve the current aqueous pore model, the molecular dynamic simulations were used.
Molecular Dynamic Models
MD simulations provide the most basic and fundamental approaches for modeling the effect of electric fields on cells. Considering the cell membrane as a lipid bilayer, the interacting particles are characterized by charged polar heads of the lipids. Thus, the structural details on the nanoscale can be included into the simulation for maximum accuracy and relevant physics. The MD models suggest that pulses of 1 ns duration or less may be ineffective at causing electro-pore formation, regardless of the field amplitude, since the field will not be present long enough to support the entire sequence of water and lipid reorganization necessary to form a stable pore[73]. MD appears to be the most plausible approach to understanding spatial and temporal electric field membrane interactions on a nanoscale. MD is a time-dependent kinetic scheme that follows the trajectories of N-interaction bodies subject to chosen external fields. It is a microscopic approach that specifically treats every atom within the chosen simulation region. MD relies on the application of classical Newtonian mechanics for the dynamical movement of ions and neutral atoms, taking into account the multiple interactions within a realistic molecular representation of the biosystem. The results of MD calculations shed light on the physics of such interactions and provide information on critical electric fields for field–membrane interactions. Thus, for example, a segment of the lipid bilayer membrane or a channel protein is first constructed taking into account the initial geometric arrangement of all the atoms and their bonding angles. Regions of water containing user-specified ion densities are then defined on either side of the membrane to form the total simulation space. This method has only recently been used to model cellular membranes under the electrical field [74-77], mechanical stress or both of them[78, 79]. Here, a few of most significant results are mentioned separately:
Behavior of Water Molecules
According to Tieleman’s investigation, qualitatively, the pore formation does not seem to depend on the nature of the lipid head-group. In fact, his MD simulations show that pore formation is driven by local electric field gradients at the water/lipid interface. The initial steps of pore formation do not seem to depend on the nature of the lipid headgroups but are determined by the increased likelihood of water defects. Indeed Water molecules move in field gradients, which increases the probability of water defects penetrating into the bilayer interior. Such water defects cause a further increase in the local electric field, accelerating the process of pore formation. The likelihood of pore formation appears to be increased by local membrane defects involving lipid headgroups. The resulting pores are hydrophilic, lined by phospholipid headgroups[80].
Formation of Water Wire and Lipid Headgroups Reorient
Formation of water wires and water channels in the hydrophobic domain of lipid bilayers when these are subject to an electrical field in the range 0.5–1.0 V/nm demonstrated by Tarek and coworker. The simulations have evidenced that the electroporation process takes place in two stages; first, water molecules organized in single file like wires penetrate the hydrophobic core of the bilayer. This water penetration is apparently favored by local defects in the lipid headgroup region. Then, these ‘defects’ grow in size, reaching the nanometer length scale, these pores are stabilized by lipid headgroups that migrate from the membrane-water interface to the middle of the bilayer lipid interface. It is suggested that water wires formation, the precursor to full electroporation, is driven by local field gradients at the water[81].
Analysis of Energetics
Reorganization of Water Dipoles
To kman et al, using molecular dynamics simulations, demonstrated that pore formation is driven by the reorganization of the interfacial water molecules. Their energetic analysis and comparisons of simulations with and without the lipid bilayer showed that the poration process is driven by field-induced reorganization of water dipoles at the water-lipid or water-vacuum interfaces into more energetically favorable configurations, with their molecular dipoles oriented in the external field. They concluded that interfacial water molecules are the main players in the process, its initiators and drivers. The role of the lipid layer, to a first-order approximation, is then reduced to a relatively passive barrier. This new view of electroporation simplifies opens up new opportunities in both theoretical modeling of the process and experimental research to better control or to use it in new, innovative ways[82]. According to this view, the pore formation is driven by the collective tendency of the interfacial water dipoles to minimize their electrostatic interactions, while adopting an orientation that minimizes the energy of the water dipole in the external electric field, reflected in the steady drop in the per molecule energy of waters in the nascent pore as the protrusion develops.
Properties of Nanopores
According to several experimental and theoretical models, the following features have been obtained for the pores:
Pore Lifetime
Studies that employed conventional electroporation (using milli- and microsecond-duration pulses) showed that the permeabilized state of the plasma membrane persists from fractions of a second to minutes and even hours after the treatment[83, 84] However, first studies of plasma membrane poration with USEP introduced nanopores as “nanosecond-duration, nanometer-diameter openings in the membrane”[85]. In the same study, observations of the effect of high-rate pulse trains indicated longer nanopore lifetime, possibly “on the order of microseconds”. Counterintuitively, the smallest pores (∼1 nm in diameter) were reported to have relatively long lifetime, probably because of the existence of a significant energy barrier which prevents their complete closing[83].
Ion Selectivity of Nanopores
Aqueous pores having diameter much larger than ions passing through them are unlikely to have any ion selectivity, and relative permeabilities of different ions will be proportional to their water mobility. In contrast, USEP-opened nanopores display preferential permeability to certain ion species. This effect was found both in cells that were exposed intact and “patched” afterward, and in those that were “patched” prior to the exposure[86]. These experiments established that nanopores are preferentially permeable to cations. Although K+ and Cl− ions have the same mobility in water, Cl− anion was about 10-fold less permeable, and this result was reproduced in several independent series of experiments[85].
Number and Size of Pore
From those studies in which the number and sizes of pores were estimated, it follows that the pores induced by exposure to an electric pulse leading to the poration of almost 100% of the cells are smaller than 0.5–1.0 nm[87, 88], Nevertheless, the appearance of such small pores is sufficient for a cell to be regarded as porated. fractions of porated cell dependence on the amplitude and the duration of the electric pulse.[89]
Kinetics of Pore Formation and Resealing
The kinetics of pore formation in cellular and artificial membranes during an electric pulse and their disappearance after the pulse are mainly measured by studying the time course of the changes of (1) the membrane conductivity[90-93], (2) the total permeability of the membrane to small inorganic ions[87, 94], and (3) the fraction of cells permeable to small inorganic ions[95, 96] or certain membrane-impermeant compounds[97]. The process of the disappearance of pores after an electric pulse is particularly important for practical applications. Estimated numerical values of the parameters show that increasing the amplitude of an electric pulse increases apparent number of pores created during the pulse (the rate of pore resealing remains the same) or the rate of pore resealing (the average number of pores remains the same)[98]. The kinetics of the membrane penetration of water fingers and of lipid headgroups is highly dependent, as expected, on the intensity of the applied field. Whereas at 0.5V/nm the first water fingers develop within a nanosecond, the same process is much faster (200 PS) for the 1.0 V/nm run. Migration of the headgroups toward the interior of the bilayer takes longer (4 ns and 1 ns, respectively for the bilayer subject to 0.5 V/nm and 1 V/nm)[81]. The resealing time could also vary with the cell type, and be affected by the composition and temperature of the extracellular medium[83].
Conclusion
Electroporation is a promising technique for targeted drug delivery with various applications in cancer therapy. The initial studies on the treatment of superficial and deep seated tumors with electroporation showed significant outcomes. To improve the electroporation as a new modality in cancer treatment, different studies should be performed to determine the mechanisms of action of the technique. Conducting in-vivo and in-vitro experimental, as well as exact modeling studies can shed light on the different aspects of mechanisms of action of this technique.
References
- Chang, D.C., et al., Guide to electroporation and electrofusion. 1992: Academic Press.
- Orlowski, S., et al., Transient electropermeabilization of cells in culture: increase of the cytotoxicity of anticancer drugs. Biochemical pharmacology, 1988. 37(24): p. 4727-4733.
- Poddevin, B., et al., Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochemical pharmacology, 1991. 42: p. S67-S75.
- Neumann, E., et al., Mechanism of electroporative dye uptake by mouse B cells. Biophysical journal, 1998. 74(1): p. 98-108.
- Tamosiunas, M., et al., Electroporation of transplantable tumors for the enhanced accumulation of photosensitizers. Acta Bio-Optica et Informatica Medica. Inżynieria Biomedyczna, 2006. 12(1): p. 57-59.
- Stampfli, R., [Permeability of the membrane of Ranvier’s node to potassium following excitation.]. Journal de physiologie, 1958. 50(2): p. 520-523.
- Coster, H., A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of “punch-through”. Biophysical journal, 1965. 5(5): p. 669-686.
- Neumann, E. and K. Rosenheck, Permeability changes induced by electric impulses in vesicular membranes. The Journal of membrane biology, 1972. 10(1): p. 279-290.
- Pucihar, G., et al., The influence of medium conductivity on electropermeabilization and survival of cells in vitro. Bioelectrochemistry, 2001. 54(2): p. 107-115.
- Miklavčič, D., et al., The importance of electric field distribution for effective in vivo electroporation of tissues. Biophysical journal, 1998. 74(5): p. 2152-2158.
- Tekle, E., R.D. Astumian, and P.B. Chock, Electro-permeabilization of cell membranes: effect of the resting membrane potential. Biochemical and biophysical research communications, 1990. 172(1): p. 282-287.
- Kotnik, T., et al., Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses: Part I. Increased efficiency of permeabilization. Bioelectrochemistry, 2001. 54(1): p. 83-90.
- Golzio, M., et al., Control by osmotic pressure of voltage-induced permeabilization and gene transfer in mammalian cells. Biophysical journal, 1998. 74(6): p. 3015-3022.
- Kotnik, T., F. Bobanović, and D. Miklavcˇicˇ, Sensitivity of transmembrane voltage induced by applied electric fields—a theoretical analysis. Bioelectrochemistry and bioenergetics, 1997. 43(2): p. 285-291.
- Wilke, N., et al., Silicon microneedle electrode array with temperature monitoring for electroporation. Sensors and Actuators A: Physical, 2005. 123: p. 319-325.
- Mali, B., et al., Tumor size and effectiveness of electrochemotherapy. Radiology and oncology, 2013. 47(1): p. 32-41.
- Neumann, E., et al., Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO journal, 1982. 1(7): p. 841.
- Heller, R., et al., Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer, 1998. 83(1): p. 148-157.
- Neal II, R.E., et al., Treatment of breast cancer through the application of irreversible electroporation using a novel minimally invasive single needle electrode. Breast cancer research and treatment, 2010. 123(1): p. 295-301.
- Gehl, J., T. Skovsgaard, and L.M. Mir, Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochimica et Biophysica Acta (BBA)-General Subjects, 2002. 1569(1): p. 51-58.
- LANDSTRÖM, F.J., et al., Electroporation therapy of skin cancer in the head and neck area. Dermatologic Surgery, 2010. 36(8): p. 1245-1250.
- Soden, D.M., et al., Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours. Cancer letters, 2006. 232(2): p. 300-310.
- Mozzillo, N., et al., Use of neoadjuvant electrochemotherapy to treat a large metastatic lesion of the cheek in a patient with melanoma. J Transl Med, 2012. 10: p. 131.
- Mir, L.M., et al., Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. British journal of cancer, 1998. 77(12): p. 2336.
- Serša, G., S. Kranjc, and M. Čemažar, Improvement of combined modality therapy with cisplatin and radiation using electroporation of tumors. International Journal of Radiation Oncology* Biology* Physics, 2000. 46(4): p. 1037-1041.
- Sersa, G., M. Cemazar, and Z. Rudolf, Electrochemotherapy: advantages and drawbacks in treatment of cancer patients. Cancer Ther, 2003. 1: p. 133-142.
- Spugnini, E.P., et al., Electrochemotherapy for the treatment of squamous cell carcinoma in cats: A preliminary report. The Veterinary Journal, 2009. 179(1): p. 117-120.
- Curatolo, P., et al., Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Annals of surgical oncology, 2012. 19(1): p. 192-198.
- Rols, M.-P., et al., Electrochemotherapy of cutaneous metastases in malignant melanoma. Melanoma research, 2000. 10(5): p. 468-474.
- Sersa, G., et al., Electrochemotherapy of mouse sarcoma tumors using electric pulse trains with repetition frequencies of 1 Hz and 5 kHz. The Journal of membrane biology, 2010. 236(1): p. 155-162.
- Rodrı́guez-Cuevas, S., et al., Electrochemotherapy in primary and metastatic skin tumors: phase II trial using intralesional bleomycin. Archives of medical research, 2001. 32(4): p. 273-276.
- Giardino, R., et al., Electrochemotherapy a novel approach to the treatment of metastatic nodules on the skin and subcutaneous tissues. Biomedicine & pharmacotherapy, 2006. 60(8): p. 458-462.
- Mir, L.M. and S. Orlowski, Mechanisms of electrochemotherapy. Advanced drug delivery reviews, 1999. 35(1): p. 107-118.
- Sersa, G., et al., Electrochemotherapy with bleomycin in the treatment of hypernephroma metastasis: case report and literature review. Tumori, 1999. 86(2): p. 163-165.
- Miklavcic, D., et al., Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online, 2010. 9(10): p. 1-12.
- Davalos, R. and B. Rubinsky, Tissue ablation with irreversible electroporation, 2011, Google Patents.
- Guo, Y., et al., Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer research, 2010. 70(4): p. 1555-1563.
- Al-Sakere, B., et al., Tumor ablation with irreversible electroporation. PloS one, 2007. 2(11): p. e1135.
- Rubinsky, B., Irreversible electroporation: implications for prostate ablation. Technology in cancer research & treatment, 2007. 6(4).
- Edd, J.F., et al., In vivo results of a new focal tissue ablation technique: irreversible electroporation. Biomedical Engineering, IEEE Transactions on, 2006. 53(7): p. 1409-1415.
- Van Wamel, A., et al., Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. Journal of controlled release, 2006. 112(2): p. 149-155.
- Longsine-Parker, W., et al., Microfluidic electro-sonoporation: a multi-modal cell poration methodology through simultaneous application of electric field and ultrasonic wave. Lab on a Chip, 2013. 13(11): p. 2144-2152.
- Wilgenhof, S., et al., Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. Journal of immunotherapy, 2011. 34(5): p. 448-456.
- Kinosita Jr, K. and T.Y. Tsong, Voltage-induced pore formation and hemolysis of human erythrocytes. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1977. 471(2): p. 227-242.
- Teissie, J. and M.-P. Rols, An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophysical journal, 1993. 65(1): p. 409-413.
- Bedlack Jr, R.S., et al., Distinct electric potentials in soma and neurite membranes. Neuron, 1994. 13(5): p. 1187-1193.
- Gehl, J., Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiologica Scandinavica, 2003. 177(4): p. 437-447.
- Teissie, J., M. Golzio, and M. Rols, Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of?) knowledge. Biochimica et Biophysica Acta (BBA)-General Subjects, 2005. 1724(3): p. 270-280.
- Chernomordik, L., et al., Breakdown of lipid bilayer membranes in an electric field. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1983. 736(2): p. 203-213.
- Chernomordik, L.V. and Y.A. Chizmadzhev, Electrical breakdown of lipid bilayer membranes, in Electroporation and Electrofusion in Cell biology. 1989, Springer. p. 83-95.
- Glaser, R.W., et al., Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1988. 940(2): p. 275-287.
- Chang, D.C., Structure and dynamics of electric field-induced membrane pores as revealed by rapid-freezing electron microscopy. Guide to electroporation and electrofusion, 1992: p. 9-28.
- Kinosita Jr, K. and T.Y. Tsong, Voltage-induced conductance in human erythrocyte membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1979. 554(2): p. 479-497.
- Jones, J., et al., Response of cultured myocardial cells to countershock-type electric field stimulation. American Journal of Physiology-Heart and Circulatory Physiology, 1978. 235(2): p. H214-H222.
- Jones, J.L., R.E. Jones, and G. Balasky, Microlesion formation in myocardial cells by high-intensity electric field stimulation. American Journal of Physiology-Heart and Circulatory Physiology, 1987. 253(2): p. H480-H486.
- Abidor, I., et al., Electric breakdown of bilayer lipid membranes: I. The main experimental facts and their qualitative discussion. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1979. 104: p. 37-52.
- Chizmadzhev, Y.A., V. Arakelyan, and V. Pastushenko, 248-Electric breakdown of bilayer lipid membranes III. Analysis of possible mechanisms of defect origination. Bioelectrochemistry and Bioenergetics, 1979. 6(1): p. 63-70.
- Benz, R., F. Beckers, and U. Zimmermann, Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. The Journal of membrane biology, 1979. 48(2): p. 181-204.
- Zimmermann, U., Electric field-mediated fusion and related electrical phenomena. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes, 1982. 694(3): p. 227-277.
- Tovar, O. and L. Tung, Electroporation and recovery of cardiac cell membrane with rectangular voltage pulses. American Journal of Physiology, 1992. 263: p. H1128-H1128.
- Mehrle, W., R. Hampp, and U. Zimmermann, Electric pulse induced membrane permeabilisation. Spatial orientation and kinetics of solute efflux in freely suspended and dielectrophoretically aligned plant mesophyll protoplasts. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1989. 978(2): p. 267-275.
- Hibino, M., H. Itoh, and K. Kinosita Jr, Time courses of cell electroporation as revealed by submicrosecond imaging of transmembrane potential. Biophysical journal, 1993. 64(6): p. 1789-1800.
- Kalinowski, S., et al., Chronopotentiometric studies of electroporation of bilayer lipid membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1998. 1369(2): p. 204-212.
- Litster, J., Stability of lipid bilayers and red blood cell membranes. Physics Letters A, 1975. 53(3): p. 193-194.
- Taupin, C., M. Dvolaitzky, and C. Sauterey, Osmotic pressure-induced pores in phospholipid vesicles. Biochemistry, 1975. 14(21): p. 4771-4775.
- Genco, I., et al., Electroporation in symmetric and asymmetric membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1993. 1149(1): p. 10-18.
- Wilhelm, C., et al., Kinetics of pore size during irreversible electrical breakdown of lipid bilayer membranes. Biophysical journal, 1993. 64(1): p. 121-128.
- Melikov, K.C., et al., Voltage-induced nonconductive pre-pores and metastable single pores in unmodified planar lipid bilayer. Biophysical journal, 2001. 80(4): p. 1829-1836.
- Kotulska, M., et al., Metastable pores at the onset of constant-current electroporation. The Journal of membrane biology, 2010. 236(1): p. 37-41.
- Pakhomov, A.G., D. Miklavčič, and M.S. Markov, Advanced electroporation techniques in biology and medicine. 2010: CRC Pr I Llc.
- Neumann, E. and S. Kakorin, Physical Chemistry Theory of Membrane Electroporation and Electrotransfer of Biogenic Agents. Advanced electroporation techniques in biology and medicine, 2010.
- Weaver, J.C. and Y.A. Chizmadzhev, Theory of electroporation: a review. Bioelectrochemistry and bioenergetics, 1996. 41(2): p. 135-160.
- Schoenbach, K.H., et al., The effect of intense subnanosecond electrical pulses on biological cells. Plasma Science, IEEE Transactions on, 2008. 36(2): p. 414-422.
- Groot, R. and K. Rabone, Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophysical journal, 2001. 81(2): p. 725-736.
- de Vries, A.H., A.E. Mark, and S.J. Marrink, Molecular dynamics simulation of the spontaneous formation of a small DPPC vesicle in water in atomistic detail. Journal of the American Chemical Society, 2004. 126(14): p. 4488-4489.
- Hu, Q., et al., Simulations of transient membrane behavior in cells subjected to a high-intensity ultrashort electric pulse. Physical Review E, 2005. 71(3): p. 031914.
- Vernier, P.T., Y. Sun, and M.A. Gundersen, Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC cell biology, 2006. 7(1): p. 37.
- Tieleman, D.P., et al., Simulation of pore formation in lipid bilayers by mechanical stress and electric fields. Journal of the American Chemical Society, 2003. 125(21): p. 6382-6383.
- Akinlaja, J. and F. Sachs, The breakdown of cell membranes by electrical and mechanical stress. Biophysical journal, 1998. 75(1): p. 247-254.
- Tieleman, D.P., The molecular basis of electroporation. BMC biochemistry, 2004. 5(1): p. 10.
- Tarek, M., Membrane electroporation: a molecular dynamics simulation. Biophysical journal, 2005. 88(6): p. 4045-4053.
- Tokman, M., et al., Electric field-driven water dipoles: nanoscale architecture of electroporation. PloS one, 2013. 8(4): p. e61111.
- Saulis, G., M. Venslauskas, and J. Naktinis, Kinetics of pore resealing in cell membranes after electroporation. Bioelectrochemistry and bioenergetics, 1991. 26(1): p. 1-13.
- Saulis, G., Kinetics of pore disappearance in a cell after electroporation. Biomedical sciences instrumentation, 1998. 35: p. 409-414.
- Thomas Vernier, P., et al., Nanoelectropulse-induced phosphatidylserine translocation. Biophysical journal, 2004. 86(6): p. 4040-4048.
- Hille, B., Ion channels of excitable membranes. Vol. 507. 2001: Sinauer Sunderland, MA.
- Serpersu, E.H., K. Kinosita Jr, and T.Y. Tsong, Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1985. 812(3): p. 779-785.
- Schwister, K. and B. Deuticke, Formation and properties of aqueous leaks induced in human erythrocytes by electrical breakdown. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1985. 816(2): p. 332-348.
- Saulis, G. and M. Venslauskas, Cell electroporation: Part 1. Theoretical simulation of the process of pore formation in a cell. Bioelectrochemistry and bioenergetics, 1993. 32(3): p. 221-235.
- Pakhomov, A.G., et al., Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochemical and biophysical research communications, 2009. 385(2): p. 181-186.
- Chernomordik, L., et al., The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1987. 902(3): p. 360-373.
- Chen, W. and R.C. Lee, An improved double vaseline gap voltage clamp to study electroporated skeletal muscle fibers. Biophysical journal, 1994. 66(3): p. 700-709.
- Pliquett, F. and S. Wunderlich, Relationship between cell parameters and pulse deformation due to these cells as well as its change after electrically induced membrane breakdown. Journal of electroanalytical chemistry and interfacial electrochemistry, 1983. 155: p. 467-475.
- Glaser, R., A. Wagner, and E. Donath, Volume and ionic composition changes in erythrocytes after electric breakdown: Simulation and experiment. Bioelectrochemistry and Bioenergetics, 1986. 16(3): p. 455-467.
- Saulis, G. and M. Venslauskas, The Electrical Breakdown Of Erythrocytes-The Estimation Of The Energy Barrier Of Pore Formation. Biologicheskie Membrany, 1991. 8(3): p. 320-330.
- Riemann, F., U. Zimmermann, and G. Pilwat, Release and uptake of haemoglobin and ions in red blood cells induced by dielectric breakdown. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1975. 394(3): p. 449-462.
- Gabriel, B. and J. Teissié, Control by electrical parameters of short-and long-term cell death resulting from electropermeabilization of Chinese hamster ovary cells. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1995. 1266(2): p. 171-178.
- Saulis, G., Pore disappearance in a cell after electroporation: theoretical simulation and comparison with experiments. Biophysical journal, 1997. 73(3): p. 1299-1309.







