Walid G. Babikr1, Mohammed H. F. Shalayel*2, Ahmed H. M. K. Abdelraheem1 and Amar B. Elhussein3
1Department of Internal Medicine, College of Medicine, Najran University, KSA.
2College of Medicine, Professor of Biochemistry, Najran University, KSA.
3College of Medical Applied Sciences, Najran University, KSA.
Corresponding Author E-mail: drmhfs@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1032
Abstract
There are limited data supporting the relationship between low levels of certain minerals with abnormal glucose metabolism in humans, this does not clearly distinguish between associations of insulin resistance and type-2 diabetes mellitus with macrominerals. A hospital based- case control study was conducted in Najran University Hospital (Najran, Saudi Arabia). The study aimed to reveal the possible impact of BMI on serum calcium and magnesium levels in type 2 diabetic patients.Patients' sera were used to measure the concentrations of sodium, potassium, chloride, calcium, phosphorus and magnesium. BMI was subsequently calculated as weight (kg) per height (m2).Pearson correlation coefficient showed a high significant negative correlation for serum magnesium with FBS, BMI, HbA1c in diabetic patients (r = -0.582, p < 0.0001, -0.234, p < 0.0001 and -0.643, p< 0.0001 respectively). Meanwhile, a significant high negative correlation between serum calcium and BMI was found in our study (r = -o.140, p < 0.038). A significant renunciative decrease was found in the levels of serum calcium and magnesium in diabetic patients by increasing BMI from normal weight, overweight, obese, to morbidly obese patients as proven by one way ANOVA (p < 0.05). It was concluded that hypocalcemia and hypomagnesemia are clinical prognostic indicators for poor glycemic control in type 2 diabetes. Hypomagnesemia and hypocalcemia in type 2 diabetic patients are impacted on by BMI. The higher BMI, the lower serum magnesium and calcium levels and hence the worse glycemic control.
Keywords
Glycosylated hemoglobin (HbA1c); Calcium (Ca); Magnesium (Mg); Body mass index (BMI)
Download this article as:| Copy the following to cite this article: Babikr W. G, Shalayel M. H. F, Abdelraheem A. H. M. K, Elhussein A. B. Impact of Body Mass Index on Hypomagnesemia and Hypocalcemia in Type 2 Diabetic Patients. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Babikr W. G, Shalayel M. H. F, Abdelraheem A. H. M. K, Elhussein A. B. Impact of Body Mass Index on Hypomagnesemia and Hypocalcemia in Type 2 Diabetic Patients. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=9289 |
Introduction
Diabetes is a chronic disease that occurs either when the pancreas could not produce enough insulin or when the body could not efficiently use the insulin it produces. The prevalence of diabetes in the age groups between 20 to 70 years worldwide was estimated to be 8.3% in 2013 and 10.1% in 2035.1
Type-2 diabetes mellitus (DM) is a syndrome characterized by disorder in metabolism and abnormally high blood glucose (hyperglycemia) resulting from low levels of insulin with or without abnormal resistance to the action of insulin.2
Type 2 diabetes mellitus, previously referred to as “non-insulin-dependent diabetes” or “adult-onset diabetes”, accounts for 90–95% of all diabetes. Type 2 diabetes mellitusencompasses individuals who have insulin resistance and usually relative (rather than absolute) insulin deficiency. At least initially, and often throughout their lifetime, these individuals may not need insulin treatment to survive.3
Logically, lack of essential elements may lead to the failure of antioxidant defense and also to glucose intolerance, both important in the progress of diabetes. Furthermore, the overload of some transition metals may be responsible for oxidative damage.4
Among all the minerals our bodies need to maintain optimal health, magnesium is a fierce contender for first place. After decades of research, it’s become increasingly evident that magnesium, the fourth most abundant mineral in the human body, is absolutely essential for regulating hundreds of biochemical processes and several physiological systems that maintain metabolic and cardiovascular health. Mounting evidence shows this macromineral is much more important than previously thought.5
Magnesium, occasionally had been called the “forgotten cation”is the second-most abundant intracellular cation and, overall, the fourth-most abundant cation. It plays a fundamental role in many functions of the cell, including energy transfer, storage, and use; protein, carbohydrate, and fat metabolism; maintenance of normal cell membrane function; and the regulation of parathyroid hormone (PTH) secretion. Systemically, magnesium lowers blood pressure and alters peripheral vascular resistance.6,7
There are limited data supporting the relationship between low levels of certain minerals with abnormal glucose metabolism in humans, this does not clearly distinguish between associations of insulin resistance and type-2 diabetes mellitus with macrominerals. Therefore, this proposed study aims to reveal the possible impact of BMI on serum calcium and magnesium levels in type 2 diabetic patients.
Method
This is a hospital based- case control study that was conducted in Najran University Hospital (Najran City, southwestern Saudi Arabia) during a 7-month period from March 2015 to September 2015. Saudi Arabia). Two hundred-twenty type 2 diabetic patients (120 males and 100 females) and 76 healthy control subjects (42 males and 34 females) were enrolled in this study and their ages ranged between 35 and 86 years. All patients were newly discovered as type 2 diabetes mellitus. Patients were clinically diagnosed as type 2 diabetic according to WHO criteria.8
Weight was measured using electronic digital scales. Height was measured using a wall-mounted stadiometer. BMI was subsequently calculated as weight (kg) per height (m2).
Exclusion criteria included patients who receive minerals’ supplements or vitamin D supplementation, renal disease, pregnancy, pre-existing parathyroid, thyroid, or calcium metabolism disorders, requirement for calcium channel blockers, type I diabetes and active malignancies.
Cases for the study were selected in accordance with the above mentioned inclusion and exclusion criteria. Data were collected using a pre-tested proforma meeting the objectives of the study.
Venous blood samples were collected following an overnight fast (>10 h) and 2-hour after 75 g glucose load in plane vacutainers and anticoagulant containing vacutainers.Blood tubes were centrifuged (2280×g, 30 min). Fasting plasma glucose and 2h-plasma glucose (after 75g glucose load) were estimated.HbA1c was measured using spectrophotometric method. The sera were used to measure the concentrations ofsodium, potassium, chloride, calcium, phosphorus and magnesium as well.
Results of this study were statistically analyzed using statistical package for social science (SPSS) program. Significant differences between groups were assessed by one-way ANOVA and t- test. Skewness and kurtosis values of variables were less than 1 indicating normal distribution of data variables.
Pearson correlation was done and the r values were calculated at level of (p < 0.05) significance.
Results
Our study included 220 type 2 diabetic patients (120 males and 100 females) admitted to Najran University hospital and 76 healthy control subjects (42 males and 34 females), their ages ranged between 35 and 86 years.
Fasting blood sugar, FBS (mmol/l) was 11.42 ±4.76 (mean ± S.D.) while 2h-BS was 14.22± 5.01. Results of the studied parameters are listed in table I.
Our results revealed that HbA1c % was significantly higher in diabetic group than that of healthy control group (9.71 ± 2.39 vs.5.47 ± 0.70, p < 0.01).
Pearson correlation coefficient showed a high significant negative correlation for serum magnesium with FBS, BMI, HbA1c in diabetic patients(r = -0.582, p < 0.0001, -0.234, p < 0.0001 and -0.643, p< 0.0001 respectively). Moreover, a significant high negative correlation between serum calcium with HbA1cand BMI was found in our study (r = -0.272, p < 0.0001 and -0.140, p < 0.038 respectively).
Results of macrominerals serum levels in diabetic and control subjects are listed in table II while, results of macrominerals in different BMI groups of type 2 diabetic patientsare listed in table III.Interestingly, there were a significant renunciative decrease in the levels of serum calcium and magnesium in diabetic patients by increasing BMI from normal weight, overweight, obese, to morbidly obese patients as proven by one way ANOVA (p < 0.05) (table III, figure 1). In addition, there was statistically significant positive correlation between levels of serum calcium with age of type 2 diabetic patients and statistically significant negative correlation between levels of serum magnesium with age of type 2 diabetic patients (r = 0.285, p < 0.0001 and -0.214, p < 0.001 respectively). Such significant correlations were not found between serum calcium and magnesium with gender.
Table I: Results of BMI, FBS, 2h-BS and HbA1c
| Subjects | BMI | FBS mmol/L | 2h-BS
mmol/L |
HbA1c % | |
| Diabetic | Mean | 34.09** | 11.42** | 14.22** | 9.71** |
| Standard Error | 1.098 | 0.321 | 2.55 | 0.161 | |
| Standard Deviation | 16.28 | 4.76 | 5,01 | 2.39 | |
| Healthy control | Mean | 25.75 | 4.31 | 6.39 | 5.47 |
| Standard Error | 0.325 | 0.071 | 0.361 | 0.081 | |
| Standard Deviation | 2.84 | 0.617 | 0.721 | ||
BMI: Body Mass Index; FBS: Fasting Blood Sugar; 2h-BS: 2 hours Blood Sugar (after 75g glucose load); HbA1c: Glycated hemoglobin.
*Significant difference as compared with control group (Significance at level p < 0.05).
**Highly significant (Significance at level of p < 0.01).
Table II: Levels of macrominerals in the studied groups
| Macrominerals | Sodium | Potassium | Chloride | Calcium | Phosphorus | Magnesium | |
|
Type-2 diabetic |
Mean (mmol/L) | 138.93 | 4.42 | 104.29 | 2.16* | 1.19 | 0.74** |
| S.D. | 2.531 | 0.450 | 7.746 | 0.252 | 0.249 | 0.106 | |
|
Control |
Mean (mmol/L) | 139.22 | 4.43 | 102.20 | 2.35 | 1.18 | 1.74 |
| S.D. | 2.347 | 0.435 | 2.857 | 0.091 | 0.254 | 0.116 |
S.D: Standard deviation
* Significant (p < 0.05).
**Highly significant (Significance at level of p < 0.01).
Table III: Results of macrominerals serum levels in different BMI groups of type 2 diabetic patients
| Sodium | Potassium | Chloride | Calcium | Phosphorus | Magnesium | |
| Normal weight
(18.5 – 24.9 Kg) |
139.59 ± 2.28 | 4.47 ± 0.48 | 103.71 ± 3.36 | 2.294 ± 0.20* | 1.166 ± 0.24 | 1.35 ± 0.49* |
| Overweight
(25 – 29.9 Kg) |
138.69 ± 2.48 | 4.42 ± 0.44 | 103.56 ± 3.84 | 2.25 ± 0.22* | 1.18 ± 0.26 | 1.167 ± 0.51* |
| Obese
(30 – 34.9 Kg) |
138.66 ± 2.74 | 4.41 ± 0.40 | 104.63 ± 3.08 | 2.18 ± 0.25* | 1.195 ± 0.26 | 0.851 ± 0.32* |
| Morbid obesity
(≥ 35 Kg) |
139.43 ± 2.18 | 4.42 ± 0.49 | 102.93 ± 12.7 | 2.13 ± 0.24* | 1.20 ± 0.24 | 0.751 ± 0.11* |
Data (mmol/L) are expressed as means ± S.D.
Na: Sodium; K: Potassium; Cl: Chloride; Ca: Calcium; P: Phosphorus; Mg: Magnesium.
* Significant differences (p < 0.05) when compared with normal control group.
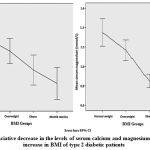 |
Figure 1: Renunciative decrease in the levels of serum calcium and magnesium by exponential increase in BMI of type 2 diabetic patients. |
Discussion
Almost all enzymatic reactions that use energy for phosphorylation need magnesium for activation. Magnesium is involved in most of biochemical metabolic pathways (e.g., deoxyribonucleic acid [DNA] and protein synthesis, glycolysis, oxidative phosphorylation). Magnesium serves as a molecular stabilizer of ribonucleic acid (RNA), DNA, and ribosomes. Because magnesium is bound to adenosine triphosphate (ATP) inside the cell, shifts in intracellular magnesium concentration may help to regulate cellular bioenergetics, such as mitochondrial respiration.9
Our study showed significant hypomagnesemia in type 2 diabetes mellitus when compared with control group (0.75 mg/dl ± 0.106 vs. 1.74 ± 0.116, p < 0.01). Hypomagnesemia was found to be associated with poor glycemic control and increased incidence of complications.10
Moreover, there was a high significant negative correlation between serum magnesium and FBS. This is in concordance with results of previous several studies that have reported an inverse relationship between glycemic control and serum Mg levels.11, 12
Although many authors have suggested that diabetes may induce hypomagnesemia and higher incidence of diabetes increases the risk for Mg deficiency13, others have reported that higher Mg intake may confer a lower risk for type 2 diabetes mellitus.14
It is interesting that the induction of Mg deficiency has been shown to reduce insulin sensitivity in individuals without diabetes, whereas Mg supplementation has been shown to improve glucose handling in elderly individuals without diabetes.15
However, some other studies did not observe a correlation between glycemic control and serum Mg levels or improvement of glycemic control with Mg replacement.16 Insulin by itself does not influence the serum magnesium concentration. But in a hyperglycemic state, insulin causes a rapid intracellular uptake of glucose. This process causes an increase in the phosphorylation by sodium-potassium ATPase on the cell membrane. Since magnesium is a cofactor for sodium potassium ATPase, magnesium is consumed, which subsequently decreases the serum magnesium concentration.16
Our interesting finding that magnesium decrease with exponential increase of BMI in type 2 diabetic patients may be explained by the link of adiposity with type 2 diabetes sinceadiposity, even in the absence of metabolic dysfunction, is a risk factor for prediabetes and type 2 diabetes17,18meanwhileoverweight and obese individuals are at increased risk for developing type 2 diabetes(compared with normal weight patients)17and the risk of type 2 diabetes increases exponentially as BMI increases above about 25 kg/m2.19Overpositive energy balance that leads to obesity, especially excess visceral adiposity, provides an adverse metabolic environment that fosters the development of insulin resistance and beta cell dysfunction resulting in the emergence of glucose intolerance and type 2 diabetes mellitus.20
Many studies found a strong association between T2 DM and hypomagnesemia and low Mg concentrations were significantly linked with poor glycemic control.21,22 Magnesium (Mg) is actively involved in a number of metabolic reactions as an important co-factor for kinase with special emphasis on carbohydrate metabolism.23 Hence, the regulatory role of magnesium in important metabolic pathways involved in energy metabolism and glycaemic control is well addressed.
Our study revealed a high significant negative correlation between serum calcium and BMI (r = -o.140, p < 0.038). Moreover, there was a significant renunciative decrease in the level of serum calcium in diabetic patients by increasing BMI from normal weight, overweight, obese, to morbidly obese patients as proven by one way ANOVA (p < 0.05). This may indebt calcium in the pathogenesis of adiposity and type 2 diabetes. Anastassios et al (2007) showed that vitamin D and calcium insufficiency may negatively affectglycemia while combined calcium and vitamin D supplementation may be beneficial in optimizing glucose metabolism.24
Calcium is essential for insulin-mediated intracellular processes in insulin-dependent tissues such as skeletal muscle and adipose tissue25 with a very narrow range of calcium ions needed for optimal insulin-mediated functions.26 Changes in calcium ions in primary insulin target tissues may contribute to peripheral insulin resistance.27Enhanced insulin sensitivity mediated through the action of 1,25-hydroxyvitamin D3 and/or osteocalcin may have emerged as an adaptation to conditions when dietary sources of calcium are scarce in order to overcome the secretory deficiency of pancreatic beta-cells induced by low calcium levels.28
In the Women’s Health Initiative Study, women who were randomly assigned to calcium and vitamin D supplementation arm had significantly less weight gain.29 Major et al., (2007) showed that calcium and vitamin D supplementation enhanced body weight loss in over weight and obese women.30 Contrarily, Chandler et al (2015) reported that in overweight African-Americans, short-term high-dose vitamin D supplementation did not alter BMI.31
Conclusion
Hypocalcemia and hypomagnesemia are clinical prognostic indicators for poor glycemic control in type 2 diabetes mellitus. Hypomagnesemia and hypocalcemia in type 2 diabetic patients are impacted on by BMI. The higher BMI, the lower serum magnesium and calcium levels and hence the worse glycemic control.
Contributors
MHFS: concept and design of the study, supervision of the study, clinical studies, analysis and interpretation, manuscript preparation, critical and final revision of the manuscript and literature search. WGB: concept and design of the study, data collection, and literature search. AHMKA: data collection, clinical studies and data acquisition. ABE: data collection, data acquisition, statistical analysis and laboratory investigations.
Acknowledgment
This work was supported by a grant from the Deanship of Scientific Research, Najran University, KSA (NU/MID/14/017).
Disclosure
None declared.
Ethics approval
All patients were assured that all their obtained information will be handled in a confidentialatmosphere and it will not affect their lifeafter taking verbal and written consent. Ethical clearance and protocol approval were obtained from research and ethics committee of Najran University, KSA. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in Brazil 2013.
References
- World Health Organization, “Diabetes,” Fact Sheet 312, 2013,http://www.who.int/mediacentre/factsheets/fs312/en/.
- Leong A, Dasgupta K, Bernatsky S, Lacaille D, Avina-Zubieta A, Rahme E. Systematic Review and Meta-Analysis of Validation Studies on a Diabetes Case Definition from Health Administrative Records. PLoS ONE. 2013; 8(10): e75256. doi:10.1371/journal.pone.0075256
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetic Care. 2015; 38(supl.1):S8-S16. doi:2337/dc15-S005.
- Valko M, Morris H, Cronin MT (2005). Metals, toxicity and oxidative stress. Curr Med Chem.2005; 12(10):1161-208.
- Seher CL. Power of Magnesium — A Macromineral That May Improve Heart Health and Stop Diabetes. Today’s Dietitian. 2011; 13 (12): 12.
- Konrad M. Disorders of magnesium metabolism. Geary D, Shaefer F. Comprehensive Pediatric Nephrology. Philadelphia PA: Mosby Elsevier; 2008. 461-475.
- Martin KJ, González EA, Slatopolsky E. Clinical consequences and management of hypomagnesemia. J Am SocNephrol. 2009; 20(11):2291-5.
- http://www.who.int/diabetes/publications/report-hba1c_2011.pdf (Accessed on June 07, 2011).
- http://emedicine.medscape.com/article/2038394-overview. Updated: Dec 12, 2014.
- Dasgupta A, Sarma D, Saikia UK. Hypomagnesemia in type 2 diabetes mellitus. Indian Journal of Endocrinology and Metabolism. 2012; 16(6):1000-1003. doi:10.4103/2230-8210.103020.
- Pham PC, Pham PM, Pham PA, Pham SV, Pham HV, Miller JM, Yanagawa N, Pham PT: Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. ClinNephrol. 2005; 63:429– 436.
- Resnick L, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM: Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia. 1993; 36 :767– 770.
- Bertinato J, Xiao C, Ratnayake W, Fernandez L, Lavergne C, Wood C, &Swist E. Lower serum magnesium concentration is associated with diabetes, insulin resistance, and obesity in South Asian and white Canadian women but not men. Food & Nutrition Research. 2015; 59. doi:http://dx.doi.org/10.3402/fnr.v59.25974.
- van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR: Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in US black women. Diabetes Care. 2006; 29:2238– 2243.
- Pham PCT, PhamPMT, Pham SV, Miller JM, & Pham PTT. Hypomagnesemia in patients with type 2 diabetes. Clinical journal of the American Society of Nephrology. 2007; 2(2): 366-373.
- Lau A, Chan L. Electrolytes, other minerals, and trace elements. In: Lee M (ed). Basic skills in interpreting laboratory data. 4th edition, American society of Health-System Pharmacists, 2009, pp. 119-149.
- Franssens BT, van der Graaf Y, Kappelle JL et al. Body Weight, Metabolic Dysfunction, and Risk of Type 2 Diabetes in Patients at High Risk for Cardiovascular Events or With Manifest Cardiovascular Disease: A Cohort Study. Diabetes Care. 2015; 38:10 1945-1951. doi:10.2337/dc15-0684.
- Gómez-Ambrosi, J., Silva, C., Galofré, J. C., Escalada, J., Santos, S., Gil, M. J., Valentí, V., Rotellar, F., Ramírez, B., Salvador, J. and Frühbeck, G. Body Adiposity and Type 2 Diabetes: Increased Risk With a High Body Fat Percentage Even Having a Normal BMI. Obesity. 2011; 19: 1439–1444. doi: 10.1038/oby.2011.36.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6.
- Day C, Bailey CJ. Obesity in the Pathogenesis of Type 2 Diabetes. British Journal of Diabetes and Vascular Disease. 2011; 11(2):55-61.
- Ghattaura N, Murphy C, Valen P. High Prevalence of Hypomagnesemia and Its Relation to BMI, Type 2 Diabetes, and Clinical Disease Measures in a VA Outpatient Rheumatology Clinic Population [abstract 2260]. Arthritis Rheumatol. 2015; 67 (suppl 10).
- Rao PP, Shariff MG. Serum Magnesium Levels in Type 2 Diabetic Patients with Microalbuminuria and Normoalbuminuria. International Journal of Scientific Study. 2015;3(4):11-15. DOI: 10.17354/ijss/2015/296.
- Mooren FC. Magnesium and disturbances in carbohydrate metabolism. Diabetes ObesMetab.2015;17(9):813-23. doi: 10.1111/dom.12492. Epub 2015 Jun 23.
- Anastassios GP, Lau J, Hu F, Dawson-Hughes B. The Role of Vitamin D and Calcium in type 2 diabetes. A systematic Review and Meta-Analysis. J ClinEndocrinolMetab. 2007; 92(6): 2017–2029.doi: 1210/jc.2007-0298.
- Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. ProcNutr Soc. 2004;63:275–278.
- Draznin B, Sussman K, Kao M, Lewis D, Sherman N. The existence of an optimal range of cytosolic free calcium for insulin-stimulated glucose transport in rat adipocytes. J Biol Chem. 1987;262:14385–14388.
- Segal S, Lloyd S, Sherman N, Sussman K, Draznin B. Postprandial changes in cytosolic free calcium and glucose uptake in adipocytes in obesity and non-insulin-dependent diabetes mellitus. Horm Res.1990;34:39–44.
- VoznesenskayaA,Tordoff Low-calcium diet prevents fructose-induced hyperinsulinemia and ameliorates the response to glucose load in rats. NutrMetab (Lond). 2015; 12: 38. doi: 10.1186/s12986-015-0035-0.
- Ref: Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Int Med. 2007; 167: 893–902.
- Ref: Major GC, Alarie F, Dore J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J ClinNutr. 2007; 85: 54–59.
- Chandler PD, Scott JB, Drake BF et al. Impact of vitamin D supplementation on adiposity in African-Americans. Nutrition & Diabetes. 2015; 5, e147. doi:10.1038/nutd.2014.44.








