Manuscript accepted on :Novemebr 20, 2009
Published online on: 18-11-2015
Plagiarism Check: Yes
Shubhangi Mhaske¹, T. Raju Ragavendra1 and C. B. S. Dangi2
¹Department of Oral Pathology, People’s Dental Academy, Bhopal India.
2Human Genetic Lab, CSRD, Peoples Group, Bhopal India.
Download this article as:| Copy the following to cite this article: Mhaske S, Ragavendra T. R, Dangi C. B. S. Areca nut as a chemical carcinogen in oral squamous cell carcinoma- A review. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Mhaske S, Ragavendra T. R, Dangi C. B. S. Areca nut as a chemical carcinogen in oral squamous cell carcinoma- A review. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=903 |
Introduction
BQ preparations
BQ preperations differ for different regions in the world. The preparation of BQ varies for different countries of world. In india, the betel quid generally contains admixture of arecanut, lime, tobacco with or without piper betel leaf (PBL)(1,4,5). In Taiwan and papua new guinea (2,3,10), instead of tobacco, piper betel inflorescence is used. Due to complex range of BQ preperations and ingredients, it is difficult to accurately assess the carcinogenic potential. It is generally having psychotropic effect owing to the presence of areca alkaloids. Four alkaloids have been conclusively identified in biochemical studies i.e. arecoline, arecadine, guacine and guacoline. Arecoline is considered to be predominant agent.
Nitrosation of arecoline leads to the formation of areca nut specific nitrosamine namely nitrosoguvacoline, nitrosoguvacine and 3-methyl nitrosomino pripionitrile, which they alkylate DNA. Metabolism of these areca nut specific nitrosamine will lead to formation of cyanoethyl, which adducts with o’methyl guanine in DNA. Prolonged exposure to this irritant leads to malignant transformation.(flowchart 1,2).
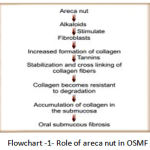 |
Flowchart 1: Role of areca nut in OSMF.
|
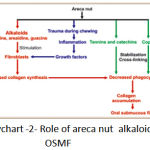 |
Flowchart 2: Role of areca nut alkaloids in OSMF.
|
Carcinogenicity, mutagenecity and genotoxicity of AN
A number of studies have been designed study the carcinogenicity of BQ ingredients in experimental animals (11-12). A direct painting of dimethy-sulfoxide ( DMSO) extract of AN in hamster cheek pouch induces early malignant changes (13). A further elevation of tumor incidence in the hamster cheek pouch is found when the concomitant application of benzopyrene and AN extract is conducted (14). In vitro studies have shown that AN extract promotes the bovine papilloma virus DNA induced malignant transformation of cultured cells. Keratinocyte inflammation
Carcinogenicity , mutagenicity and genotoxicity of areca alkaloids `
Inflammatory mediators released by the affected keratinocytes has been shown to be a critical step in tumor promotion.(15-16.)AN extracts upregulates cyclo oxygenase -2 (cox-2)m RNA and protein production, indicating the roles of Cox-2 in BQ- chewing related oral mucosal diseases.This may explain why Cox -2 mRNA expression and protein production for head aand neck tumor tissues are higher than healthy tissues(17)
The tannin and polyphenol derivatives of areca are believed to be the potential active carcinogens involved in the BQ –induced tumors . The nitrosamines are namely 3-(methyl-nitrosoamino)propionitrile (MNPN),3-(methyl-nitrosamino)-propionaldehyde(MPNA),N-nitrosoguvacoline(NG) and N-nitrosoguvacine(NGC) [1] .DNA methylation is caused by MNPN.(18)..
ROS and areca alkaloids
Reactive oxygen species (ROS) are free radicals that contain the oxygen atom. ROS form as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling. However, during times of environmental stress (e.g. UV or heat exposure)or due to carcinogen exposure , ROS levels can increase dramatically, which can result in significant damage to cell structures. This cumulates into a situation known as oxidative stress. ROS are said to be generated due to the polyphenols and tennins to in areca alkaloids.
Harmful effects of reactive oxygen species on the cell are most often:
damage of DNA
oxidations of polydesaturated fatty acids in lipids (lipid peroxidation)
oxidations of amino acids in proteins
oxidatively inactivate specific enzymes by oxidation of co-factors
The polyphenols have been suggested to show metabolic acticvation of the activation to form reactive intermediates that bind to DNA.that supposedly lead to cancer formation.(11).
Morever it is also suggested that An supprese the mutagenicity of 2-amino-3-methylimidazole(4,5).
AN extract also induces cytogenetic effects as shown by the chromosomal abberrations in cultured Chinese hamster ovary cells(19).Oral keratinocyte and fibroblasts are the major target cells of BQ attack by its ingrdients.It induces cytotoxity,DNA strand breakage,DNA protien cros linkage and unscheduled DNA synthesis (UDS) of oral keratinocytes and fibroblasts .AN extract also inhibits the DNA repair process and it also induces the terminal differentiatiation of buccal keratinocytes.(20). This further leads to GSH depletion and no concomitant increase in glutathione levels, (GSSG) (21).The alteration of these metabolic levels modulate the host suseptibility to other carcinogens as well.(1,4,5)
ROS produced during betel chewing have been demonstrated to elicit multiple detrimental upon oral mucosa.Firstly , ROS can be directly involved in the tumor initiation process by inducing genotoxicity and gene mutation[22,23,24].Secondly ROS are able to attack salivary protiens and the oral mucosa; leading to a structural change in the oral mucosa.such a change facilitating the penetration by other BQ ingredients and environmental toxicants[61thirdly inflammaotry cell infiltration has been regularly observed, [25,26,27,28,29].Activated inflammatory cells have been shown to release ROS, such a release leading to the mutation of the adjacent cells[30,31]and tumor promotion[32].
An extract has been shown to induce PGE2 production , a compound that can mediate oral mucosal inflammatory responses.Chewers usually powdered lime to the chewed An with piper betel inflorescence in the corner of the mouthleading to an elevation of the salivary pH value to around 9.9.Due to the presence of lime(Ca(OH)2 ) as a major component in BQ preparation, BQ-chewers’ saliva typically changes from an approximately neutral to an alkaline conditions and induce the mitotic conversion of cells.[74]the ROS are also capable of inducing nucleotide modificatio and the formation of 8-hydroxydeoxyguanosine.this compund formation has been stated as the biomarker of chemical carcinogenesis .
Conclusion
The most important and decisive event of chemical carcinogenesis is the interaction between resumed carcinogens and cellular macromolecules such as DNA, proteins and lipids (11,12). The oral mucosal epitehelial cells are subjected to continuous attack of genotoxic agents present in BQ, tobacco, alcohol or nitosamines and ROS (1,4,5). Antioxidants such as GSH and superoxide dismutase form conjugates with ROS and degrades reactive toxic species and interacting with the critical cellular macro molecules. Thus repeated and continuous exposure of oral mucosal cells to BQ ingredients lead to impairement of cellular- defence system. The DNA damage cells are subsequently induced by proliferative agents to replicate, DNA damage will remain permanently in the cells, leading to the formation of mutated “initiated” cells (11,12). Further promotion and progression of such mutated cells lead to the occurrence of oral precancer and cancerous lesions.
References
- ut related compounds in cultured human buccal epithelial cells. Cancer Research 1989;49;5294-8
- The involvement of reactive oxygen species (ROS) in oral cancers of betel quid/ tobacco chewers.Mutat Res 1989;214;47-61
- Nair UJ et al .Formation of IARC- IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans 37,IARC, Lyon, 1985
- Thomas SJ,Maclennan R.Slaked lime and betel nut cancer in Papua New Guinea.Lancet1992;340;577-8
- Ko YC,Huang YL,Lee CH,Chen MJ,Lin LM,Tsai CC.Betel Quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med 1995;24;450-3
- Sen S,Talukder G,Sharma A. Betel cytotoxicity.J Ethno-pharmacol 1989;26:217-47
- Sharan RN.Association of betel nut with carcinogenesis;Cancer J 1996;9:13-19
- Thomas S, Wilson A. Aquantitative evaluation of the etiological role of betel quid in oral carcinogenesis.Oral oncol 1993;29(B):265-71
- Thomas S,Kearsley J. Betel quid and oral cancer- A review; Oral oncol 1993;29(B) 251-5
- Warnakulasurya S. The role of betel quid in oral carcinogenesis.In:Usage and health issues,centre for trans –cultural oral health, London 1995.pg 61-9.
- Gupta PC, Pindborg JJ,Mehta FS.comparison of carcinogenicity of with and without tobacco:An Epidemological review. Ecology Disease 1982;1:213-9
- Health and vital statistics. Department of Health , Taiwan, Republic of China, 1999.
- Cohen SM, Elvin LB. Genetic errors, cell proliferation and carcinogenesis. Cancer Res 1991;51:6493-5
- Hursting SD, Slaga TJ, Fisher SM, DiGiovanni J, Phang JM. Mechanism based cancer prevention approeaches: targets, examples and the use of transgenic mice. J Natl Cancer Inst 1999;91:215-25.
- Ranadive KJ, Gothoskar SV, Rao AR,Tezabwala BU,Ambaye RY.Experimental stuies on betel nut and tobacco carcinogenicity.Int J Cancer1976:17:469-76
- Rao AR.Modifying influence of betel quid ingredients on BQ induced carcinogenesis in the buccal pouch.Int J Cancer 1984:33:581-6
- Barker JNWN et al . Keratinocytes as initiators of inflammation .Lancet 1991,337:211-4
- Parsonnet J. Molecular mechanism of inflammation –Promoted carcinogenesis of cancer.Cancer Res 1997;57:3620-4
- Chan G, Boyle JO, Yang EK,Zang F,SacksPG,Shah JP , et al cyclooxygenase- 2 expression is upregulated in squamous cell carcinoma of the Head and Neck.Cancer Res 1999:59:991-4
- Wenke G, Riverson A,Hoffmann D. A study of betel quid carcinogenesis 1984;5:1137-40
- Dave BJ,Trivedi AH et al. In vitro genotoxic effects of areca nut extract and arecoline .J Cancer Research Oncol 1992: 118:283-8
- Sundqvist K,Graftstrom RC. Effects of areca nut on growth and differentiation and formation of DNA damage in cultured human buccal epithelial cells.Int J Cancer 1992;52:305-10
- Sundqvist K,Liu Y,et al.Cytotoxic and genotoxic effects of areca nreactive oxygen species and of 8-OH-dG in DNA in vitro with betel quid ingredients.Chem-Biol Interact 1987;63;157-69
- Nair UJ et al. Effect of lime composition on the formation of reactive oxygen species from areca nut extract in vitro. Carcinogenesis1990:11;2145-8
- Maghji S, Warnakulasurya s.Oral submucous fibrosis, An expert symposium.Oral disease 1997;3:276-97
- Cox SC,Walker DM. OSMF –A review. Aust Dent J 1995; 41:294-9
- Riechart PA, Philipsen HP. Betel chewers mucosa – A review. J Oral Pathol Med 27 ;239-42
- Sirsat SM, Pindborg JJ, Subepithelial changes in OSMF -. Acta Pathol Microbiol Scand 1967;70:161-73.
- Mani NJ. Studies on OSMF. J Oral Med 1977;32:70-4.
- Weitberg AB et al.Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells.New Engl J Med 1983;308;26-30.
- Shacter E et al. Activated nuetrophils induce prolonged DNA damage in nieghbouring cells.Carcinogenesis 1988;9:2297-300
- Cerutti PA. pro oxidant states and tumor promotion.Science 1985;227:375-81
- Jeng JH et al. Areca nut extract upregulates prostaglandin production, cyclooxygenase 2, mRNA and protein expression of human oral keratinocytes.Carcinogenesis, 2000;21:1365-70







