J. Kausalya*, S. Padmapriya, V. Vaijayanthi, S. K.Umadevi, Sreejith and B. Senthilnathan.
Vels College of Pharmacy, Pallavaram, Chennai- 117 India.
Abstract
The objective of this study was to develop extended release matrix tablet of Tramadol hydrochloride using different combinations of hydrophilic polymers (HPMC, Carbopol, Xanthum Gum). The matrix tablets were prepared by direct compression technique. The pre and post compression characterization of blend and tablet were evaluated. The invitro dissolution studies revealed that the combination of HPMC and Carbopol in different concentration showed retard release of 65% at the end of 12th hour and more than 95% at the end of 24th hour, thereby showing good release pattern necessary for ER tablet and exhibited good kinetic release coupled of diffusion and erosion. Hence hydrophilic polymer HPMC and Carbopol was found to extended release of highly water soluble drug, Tramadol hydrochloride.
Keywords
Tramadol hydrochloride; hydrophilic polymer; extended release matrix tablet
Download this article as:| Copy the following to cite this article: Kausalya J, Padmapriya S, Vaijayanthi V, Umadevi S. K, Sreejith, Senthilnathan B. Formulation and Evaluation of Extended Release Matrix Tablet of Tramadol Hydrochloride using Hydrophilic Polymer. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Kausalya J, Padmapriya S, Vaijayanthi V, Umadevi S. K, Sreejith, Senthilnathan B. Formulation and Evaluation of Extended Release Matrix Tablet of Tramadol Hydrochloride using Hydrophilic Polymer. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=872 |
Introduction
The aim of oral sustained drug delivery system is to achieve a prolonged therapeutic effect by continuously releasing medicament over an extended period of time after administration of a single dose. Sustained release constitutes any dosage form that provides medication over an extended time period. In general, the sustained release dosage form is to maintain therapeutic blood or tissue level of drug for a prolonged period usually accomplished by attempting slow first order fashion.
In recent years sustained release dosage forms continuous to draw attention in the field of research for improved patient compliance and decreased incidence of adverse drug reaction.
Systems that are designed as prolonged release can also be considered as attempts at achieving sustained-release delivery. Repeat action tablets are an alternative method of sustained release in which multiple doses of drug are contained within a dosage form, and each dosage is related to a periodic interval. Delayed release systems, function by maintaining the drug within the dosage form for some time before release. Commonly the release rate of drug is not altered and does not result in sustained delivery once drug release has begun.
Successful fabrication of extended release products is usually difficult & and involves consideration of physicochemical properties of drug, pharmacokinetic behavior of drug, route of administration, disease state to be treated and, most importantly, placement of the drug in dosage form total will provide the desired temporal and spatial delivery pattern for the drug.
The slow first order release obtained by an extended release preparation is generally achieved by the release of the drug from a dosage form. In some cases, this can be achieved by retarding the release of drug from a dosage form and in some cases; this is accomplished by a continuous release process1.
One of the least complicated approaches to the manufacture of sustained release dosage forms involves the direct compression of the blends of drug, retardant material and additives to form a tablet in which the drug is embedded in a matrix core of the retardant2. There are various methods for preparing matrix tablets, namely Direct compression, Wet granulation, Melt granulation, Response surface methodology etc. For pain and inflammation, anti-inflammatory agents should be frequently administered to control inflammation level, Tramadol (µ opioid) may be benifited to patients experiencing pain throughtout the dosing interval3.
In the present study, matrix extended release tablets of Tramadol Hydrochloride were prepared using different polymers like Ethylcellulose, HPMC, Carbopol and Xanthum Gum resulting in less frequent fluctuations in plasma concentrations.
Materials and Methods
Tramadol Hydrochloride was provided by Milton Labs (Pondycherry, India). HPMC was supplied by Rankem limited (India), Carbopol and Xanthum Gum provided by Himedia (India), magnesium stearate and aerosol were purchased from S.D. Fine Chemicals (India).
Preparation and Characterization of matrices
A total of nine formulations of Tramadol Hydrochloride ER tablet were punched by direct compression method using different polymeric ratio of HPMC, Carbopol and Xanthum Gum, then subjected to preformulation studies like Angle of repose, Bulk density, Tapped density, Carr’s Index and post compression parameters like thickness, Hardness, Weight variation, Friability, Drug content analysis and Invitro dissolution studies.
Determination of Drug content
Tramadol Hydrochloride matrix tablets were tested for their drug content. Twenty tablets were finely powdered; 400 mg of powder was accurately weighed and transferred to a 50 ml volumetric flask. Then the volume was made up with 0.1N HCl and shaken for 10 minutes to ensure complete solubility of the drug. The mixture was centrifuged (type: 2000, Clements, Rydalmere, Australia) and 10ml of the supernatant liquid was diluted 20 times with 0.1N HCl, and after centrifugation the absorbance was determined spectrophotometrically (UV-Visible 1240 CE, Shimadzu Corp, Kyoto, Japan) at 272.8 nm.
Invitro Drug Release Studies
The matrix tablets were subjected to the paddle dissolution method using 900ml of phosphate buffer solution PH 7.4 + 0.2 as dissolution medium. The dissolution test was performed at 100rpm and temperature was set at 37 ± 1ºC. At predetermined time intervals over an 8 hour period, 4 ml samples were withdrawn, centrifuged and assayed spectrophotometrically at 272.5 nm11. After each sampling, equal volume (4ml) of fresh Phosphate Buffer with the same temperature was replaced. All experiments were run 3 times, and the calibration curve specifications were y= 0.006X + 0.005 (r2 = 0.9998, n=9).
Mechanism of drug release
To study the release mechanism, various dissolution models were applied to the invitro release profiles of the best formulations. The kinetic models include zero order, first order, higuchi’s and korsmeyer model4.
Table 1: Formulation Of Tramadol Hydrochloride Matrix Tablets Using Different Ratios Of Polymers (F1-F9).
| S. No. |
INGREIDENT
(mg) |
FORMULATION | ||||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | ||
| 1 | Tramadol hydrochloride | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 | HPMC | 50 | – | – | 30 | 20 | 30 | 20 | – | – |
| 3 | Carbopol | – | 50 | – | 20 | 30 | – | – | 30 | 20 |
| 4 | Xanthan Gum | – | – | 50 | – | – | 20 | 30 | 20 | 30 |
| 5 | MCC | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| 6 | Aerosil | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 7 | Magnesium steareate | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Total Weight | 360 | 360 | 360 | 360 | 360 | 360 | 360 | 360 | 360 | |
Table 2: Release Kinetic Data.
| Formulation | Zero order | First order | Higuchi’s equation | Korsmeyer |
| F 1 | 0.813 | 0.625 | 0.942 | 0.961 |
| F 2 | 0.917 | 0.591 | 0.977 | 0.965 |
| F 3 | 0.820 | 0.660 | 0.939 | 0.972 |
| F 4 | 0.888 | 0.613 | 0.982 | 0.963 |
| F 5 | 0.936 | 0.634 | 0.995 | 0.950 |
| F 6 | 0.895 | 0.751 | 0.954 | 0.960 |
| F7 | 0.819 | 0.631 | 0.929 | 0.959 |
| F8 | 0.908 | 0.761 | 0.967 | 0.955 |
| F9 | 0.881 | 0.690 | 0.971 | 0.969 |
Results and Discussions
Drug Excipient Compatibility Studies were carried out which showed no interaction between the drug and the polymer. The pre compression parameters like angle of repose (230 to 270), bulk density (0.57 g/ml to 0.66 g/ml), tapped density (0.54 g/ml to 0.65 g/ml) and carr’s index of (17.2-19.7) were found to be in satisfactory range. The post compression parameters like hardness was found to be in the range of 6.2 to 6.5 kg/cm2, thickness was found to be 4.02-4.62 mm, weight variation (< 5%) and friability (1%) and drug content (>95%) complied with the IP standard. The invitro dissolution studies were carried out in 0.1N HCl and PH 7.4 Phosphate buffer for 24 hours and it was found that combination of HPMC and Carbopol showed a retarded release of 63.8% at the end of 12th hour and 98.24% at the end of 24th hour. The mechanism of drug release was diffusion coupled with erosion.
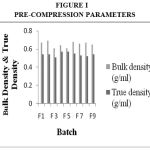 |
Figure 1:Pre-Compression Parameters. |
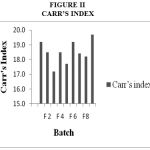 |
Figure 2:Carr’s Index
|
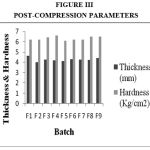 |
Figure 3:Post-Compression Parameters
|
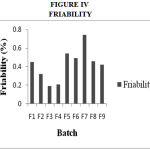 |
Figure 4: Friability.
|
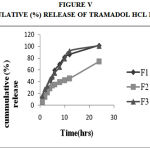 |
Figure 5: Cummulative (%) Release Of Tramadol Hcl F1,F2,F3
|
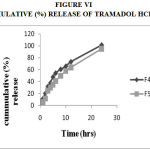 |
Figure 6: Cummulative (%) Release Of Tramadol Hcl F4,F5
|
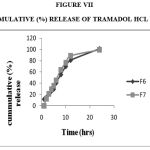 |
Figure 7: Cummulative (%) Release Of Tramadol Hcl F6,F7.
|
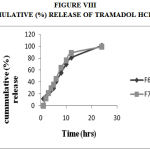 |
Figure 8: Cummulative (%) Release Of Tramadol Hcl F8,F9.
|
Conclusion
It was concluded that different polymeric combination of Hydrophilic HPMC & Carbopol polymer resulted in extending the release from the polymeric core.
Refrencess
- Remingtons, Pharmaceutical Sciences, 1980, 16th Edition, Mack Publishing Company: 1677-1683.
- Leon Lachmann, Theory and Practice of Industrial Pharmacy, 3rd Edition, Varghese Publishing House: 430-456.
- M.Dhasmana, w. rating and b. lachmann, 1990, therapeutic application of Tramadol in the treatment of pain compared to other opiates indian journal of pharmacology 22: 184-191.
- Reddy KR, Srinivas Mutalik and Srinivas Reddy, 2003. Preparation of once-daily sustained release matrix tablets of nicorandil. AAPS Pharm sci tech; 4(4), 61.
- Theophilus J. Gana , Maria Luz G. Pascual , Rosa Rosanna B. Fleming , Jeff R. Schein, Carmela C. Janagap, Jim Xiang, and Gary J. Vorsanger, 2006, Extended-release Tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind Current Medical Research and Opinion® Vol. 22, No. , 1391–1401.
- Rogelio Espinoza-Ramos, Leopoldo Villafuerte-Robles, 1999, Influence of admixed lactose on pelanserin hydrochloride release from hydroxypropylmethylcellulose matrix tablets Pharmaceutica Acta Helvetiae 74. 65–71.
- Tiwari SB, Murthy TK, Pai MR, Mehta PR, Chowdary PB, 2003, Controlled release formulation of Tramadol Hydrochloride using hydrophilic and hydrophobic matrix system. AAPS Pharm. Sci. Tech; 4:E31.
- Obaidat, 2001. Controlled release of Tramadol Hydrochloride from matrices prepared using glyceryl behenate, Eur. J. Pharm. Biopharm; 52(2),pp 231-235.
- Ryoichi Morita, Ritsuko Honda, Yoshiteru Takahashi, 2000, Development of oral controlled release preparations, a PVA swelling controlled release system (SCRS). II. In vitro and in vivo evaluation, Journal of Controlled Release 68 115–120.
- Zhi-Yan Zhanga, Qi-Neng Pinga, Bin Xiaob, 2000, Microencapsulation and characterization of Tramadol–resin Complexes Journal of Controlled Release 66, 107–113.
- Dale L. Munday Philip J. Cox, (2000), compressed xanthan and karaya gum matrices: hydration, erosion and drug release mechanisms, International Journal of Pharmaceutics 203, 179–192.
- Yihong oiu, kolette flood, kennan marsh, Stephen, 1997, design of sustained-release system for a highly water-soluble compound, ABT-089, International Journal of Pharmaceutics 203, pp. 45-54.
- Lutfi Genc, Hadi Bilac¸, Erden Gu¨ler, 1999, Studies on controlled release dimenhydrinate from matrix tablet Formulations Pharmaceutica Acta Helvetiae 74, 43–49.
- De Brabander, C. Vervaet, L. Fiermans, J.P. Remon, 2000, Matrix mini-tablets based on starch: microcrystalline wax mixtures, International Journal of Pharmaceutics 199, pp.195–203.
- M. Talukdar, G. Van den Mooter, P. Augustijns, T. Tjandra-Maga,
N. Verbeke, R. Kinget 1998, In vivo evaluation of Xanthan Gum as a potential excipient for oral controlled-release matrix tablet formulation, International Journal of Pharmaceutics 169, pp.105–113. - Mitsuo Muramatsu A, Ken Kanada A, Ayumu Nishida A, Kiyohisa Ouchi A, Noriyasu Saito A, Minoru Yoshida B, Akihiro Shimoaka B, Tetsuya Ozeki C, Hiroshi Yuasa C, Yoshio Kanaya, 2000, Application of Carbopol to controlled release preparations I.Carbopol as a novel coating material, International Journal of Pharmaceutics 199, pp.7–83.
- Steendam, C.F. Lerk, 1998, Poly (DL-lactic acid) as a direct compression excipient in controlled release tablets. Part I. Compaction behaviour and release characteristics of Poly (DL-lactic acid) matrix tablets, International Journal of Pharmaceutics 175, pp. 33–46.
- Uttam Mandal, Veeran Gowda, Aninesh Ghosh and Tapan Kumar Pal, 2007, The Pharmaceutical Socity of Japan 127 (8) pp 1281-1290.
- Mongkol Sriwongjanya, Roland Bodmeier, 1998, Effect of ion exchange resins on the drug release from matrix tablets, European Journal of Pharmaceutics and Biopharmaceutics 46, pp. 321–327.
- C.Gohel, R.K.parikh, M.N.padshala, 2007, formulation of directly compressible Isoniazid modified release matrix tablet, Indian journal of pharmaceutical sciences, pp. 640-645.
- Jaber Emami, Zhu Jiabi, Naser Tavakoli 2004, formulation and evalution of matrix tablet of lithium carbonate, AAPS PharmSciTech, 1 (4) article 34, pp.171-179.
- C Basak, Jayakumar Reddy, Lucas Mani, 2006, Formulation and Release Behaviour of Sustained Release Ambroxol Hydrochloride HPMC Matrix Tablet, IJPS vol 68, (5) pp. 594-598.
- Jaleh Varshosaz, Naser Tavakoli, and Fatemeh Kheirolahi, 2006, Use of Hydrophilic Natural Gums in Formulation of Sustained-release Matrix Tablets of Tramadol Hydrochloride, AAPS PharmSciTech; 7 (1) Article 24 pp E1-E7.
- Aulton M.E, Pharmaceutics- The Sciences of Dosages form design, international student Edition, Churchill Livingston: 129-191.







