V. Ravichandiran1, K. Masilamani2, B. Senthilnathan2, S. Hariprasad1 and D. Joseprakash2
1Vels college of Pharmacy, Pallavaram, Chennai India.
2Department of Pharmaceutics, Vels college of Pharmacy India.
Corresponding Author E-mail:sen03mpharm@gmail.com
Abstract
The aim of the present study was to prepare and characterize mouth dissolving tablet of Diltiazem hydrochloride (taste masked) using super disintegrants like (sodium starch glycolate and crosspovidone)and developing a suitable dosage form, exhibiting a maximum disintegration in the mouth in order to provide good or dispersible formulation. The result of Optimization of drug resin cross linking is done with parameters 1:4 as drug resin concentration ratio, stirring time 6hrs which showed 96.06% drug loading on to resin. Direct Compression method was selected to make the formulation. The granules so obtained were having good flow properties and tablets had shown satisfactory results with respect to physical parameters like hardness and friability, weight variation and disintegration. Disintegration results shown that the tablets containing SSG had much more effect on disintegration with respect to concentration, where as CP also shown good disintegration ability within the time limit but lesser effect compared to SSG. Dissolution results showed that tablet with good disintegration showed much better release than the other formulation used for the same purpose. Whereas with the same weight gain as that of high resin: drug concentration cross linked tablets showed the release 99.98% in 0.1NHCL.Among mouth dissolving formulation F6, was suited formulations having best drug release in 0.1NHCL. The results obtained were shown almost above 80% of drug release in 30 minutes. As per dissolution profile F6 was concluded as best formulation.
Keywords
Diltiazem hydrochloride; Granulation Process; Direct compression process
Download this article as:| Copy the following to cite this article: Ravichandiran V, Masilamani K, Senthilnathan B, Hariprasad S, Joseprakash D.Formulation Development and Evaluation of Diltiazem Hydrochloride Oral Disintegrating Tablets. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Ravichandiran V, Masilamani K, Senthilnathan B, Hariprasad S, Joseprakash D.Formulation Development and Evaluation of Diltiazem Hydrochloride Oral Disintegrating Tablets. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=817 |
Introduction
Fast-dissolving/disintegrating tablets (FDDTs) are a perfect fit for all of these patients. FDDTs disintegrate and/or dissolve rapidly in the saliva without the need for water. Some tablets are designed to dissolve in saliva remarkably fast, within a few seconds, and are true fast-dissolving tablets. Others contain agents to enhance the rate of tablet disintegration in the oral cavity, and are more appropriately termed fast-disintegrating tablets, as they may take up to a minute to completely disintegrate. The target populations for these new fast-dissolving/disintegrating dosage forms have generally been paediatric, geriatric, and bedridden or developmentally disabled patients. The basic approach used in development of MDT is the use of super disintegrants like sodium starch glycolate, crosspovidone which provide instantaneous disintegration of tablet a after putting on tongue, thereby releasing the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drugs in oral cavity and also due to pre-gastric absorption of saliva containing dispersed drugs that pass down into the stomach. Moreover, the amount of drug that is subject to first pass metabolism is reduced as compared to standard tablets.
Materials And Methods:
Diltiazem hydrochloride(Totally six formulations F1-F6) were prepared by direct compression technique with various ratios of sodium starch glycolate and crosspovidone. Indion 204,(crosslinking agent) Povidone,(binder) Methylene di chloride, Lactose, Sodium starch glycolate, Crospovidone(super disintegrants) Magnesium stearate, Talc and Aspartame.Preformulation studies of pure drug and mixed blend evaluated found were carried out using organoleptic character,angle of repose ,bulk density,tapped density,carr’s index,and fourier transform spectroscopy. After compression the tablets were evaluated for thickness, hardness, friability, weight variation, drug content uniformity and invitro dissolution studies as per the standards.
Preparation of oral disintegrating tablets
The optimized batch parameter after evaluatiion of drug entrapment is used for preparation of resinate.resinate for the formulations F1-F6 were prepared with 1:4 drug resin ratio.resinate prepared was filtered through whatman filter paper and washed with deionised water to remove unbounded drug, the resinate equivalent to 30mg of diltiazem hydrochloride (156.3mg) was weiged and granulated.lactose is mixed with the wet complex,dried mass shifted through sieve no#60. And remaining ingridients mixed properly.the dried granules and lubricant were transferred in to double cone blender,and it was mixed properly.and get fast dissolving tablets of diltiazem hydro chloride.
Characterization of Tablets
The properties of the compressed tablet, such as thickness, hardness, friability, weight variation and content uniformity were determined using reported procedure.
In vitro release studies:( F1-F6)
The dissolution test for diltiazem Hcl dispersible tablets is performed in triplicate using USP 24 paddle (Type II) method. The medium is 900 ml of 0.1N Hcl for 1hour maintained at a temperature of 37° C ± 0.5° C. The paddles are rotated at 50 rpm. 10 ml of sample are withdrawn at an interval 5, 10, 15, 20, 25, 30, 45, 60min and replaced with the same amount of 0.1N Hcl to maintain the perfect sink conditions. The percent release is calculated by using U.V.Spectrophotometer.
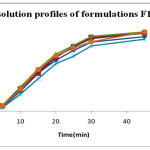 |
Figure 1:
|
Table 1:
| Time (min) | Cumulative %Drug released | |||||
| FI | F2 | F3 | F4 | F5 | F6 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 5.2 | 6.4 | 7 | 6.55 | 4.23 | 6.42 |
| 10 | 23.8 | 26.5 | 28.45 | 27.28 | 20.26 | 25.81 |
| 15 | 45.6 | 44.7 | 50.25 | 46.58 | 38.49 | 49.5 |
| 20 | 60.75 | 63.88 | 67.77 | 65.44 | 56.45 | 65.95 |
| 25 | 72.68 | 76.89 | 78.34 | 76.73 | 65.37 | 74.83 |
| 30 | 82.56 | 87.98 | 88.92 | 86.36 | 77.69 | 82.74 |
| 45 | 88.93 | 93.36 | 94.35 | 92.97 | 85.69 | 94.41 |
| 60 | 91.67 | 93.12 | 95.02 | 96.26 | 97.96 | 99.98 |
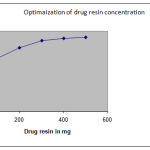 |
Figure 2:
|
Table 2: Optimization of diltiazem-indion204 complexation.
| Drug: resin | Drug loading in %w/w |
| 1:1 | 66.20±0.26 |
| 1:2 | 79.53±0.06 |
| 1:3 | 88.68±0.76 |
| 1:4 | 96.06±0.38 |
| 1:5 | 92.56±0.56 |
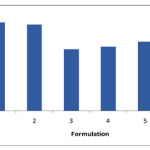 |
Figure 3:
|
The drug loading in various Drug: resin concentration was tabulated
Table 3: Evaluation Of Diltiazem Hydrochloride Granules.
| Formulations | Angle
Of repose (degrees) |
Bulk Density
(gm/ml) |
Tapped Density
(gm/ml) |
% Compressibility
|
Hausner’s
Ratio % |
| F1 | 12029”± 0.270
|
0.425±1.295 | 0.492±0.915 | 14.68±1.759 | 1.157±0.056 |
| F2 | 15030”±0.813 | 0.482±0.48 | 0.601±0.974 | 16.02±1.245 | 1.246±0.128 |
| F3 | 12029”±0.270 | 0.425±1.295 | 0.492±0.915 | 14.68±1.759 | 1.157±0.056 |
| F4 | 12029”±0.270 | 0.425±1.295 | 0.492±0.915 | 14.68±1.759 | 1.157±0.056 |
| F5 | 10028”±0.257 | 0.458±0.421 | 0.576±0.730 | 15.920±1.350 | 1.257±0.164 |
| F6 | 12029”±0.270 | 0.425±1.295 | 0.492±0.915 | 14.68±1.759 | 1.157±0.056 |
Mean ± S.D (n=3)
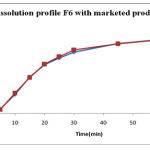 |
Figure 4:
|
Table 4: Evaluation Of Diltiazem Hydrochloride Tablets.
| Formulation | Weight
Variation (mg) |
Hardness
(Kg/cm2) |
Friability
(%) |
Thickness (mm) |
Uniformity of
Content (%) |
Water absorption ratio (%) | Disintegration Time
(seconds) |
| F1
|
298±1.35 | 3.7±0.98 | 0.65±0.89 | 3.15±0.23 | 88.68±0.14 | 88.91±0.96 | 36 |
| F2 | 297±0.97 | 3.7±0.19 | 0.76±0.04 | 3.23±0.12 | 90.48±0.13 | 90.48±0.59 | 35 |
| F3 | 299±0.43 | 3.7±0.16 | 0.80±1.2 | 3.43±0.54 | 93.62±0.23 | 92.58±0.45 | 25 |
| F4 | 301±1.56 | 3.2±1.16 | 0.75±0.69 | 3.11±0.43 | 95.23±0.06 | 91.98±0.49 | 26 |
| F5 | 296±1.75 | 3.5±0.26 | 0.66±0.48 | 3.41±0.13 | 97.57±0.01 | 93.32±0.48 | 28 |
| F6 | 299±1.09 | 3.6±0.64 | 0.74±0.18 | 3.51±0.25 | 98.83±0.02 | 95.25±0.75 | 22 |
Mean ± S.D (n=3) Comparison of Disintegration time
Results and Discussion
Optimization of diltiazem-indion204 complexation:
Diltiazem hydrochloride was loaded on Indion -204 by batch process. Complexation between drug and resin was essentially a process of diffusion of ions between the resin and surrounding drug solution. As reaction is an equilibrium phenomenon, maximum efficiency is best achieved by batch process. Also, higher swelling efficiency in batch process results in more surface area for ion exchange.
Evaluation Of Diltiazem Hydrochloride Granules
Physical evaluation
The bulk density, tapped density, compressibility index and Hausner’s Ratio were observed reveals that all formulations’ granules has excellent flow characteristics and flow rate than the raw material.
Evaluation Of Diltiazem Hydrochloride Tablets By U.V
Formulations of uncoated tablets were evaluated for physical appearance, weight variation, thickness, hardness, and friability and disintegration time. Physical parameters of tablets of each batch .Formulation F1, F2, F3, F4, F5, F6 had shown following amount of drug content 98.72, 98.32, 99.12, 98.76, 94.96, 99.38 respectively. The formulations almost had shown similar release.
Comparison Of Dissolution Profile Of F6 With Marketed Product
The dissolution test for diltiazem Hcl dispersible tablets is performed in triplicate using USP 24 paddle (Type II) method. The result obtained were compared with that of the marketed product and showed good correlation
Stability Studies
The fabricated mouth dissolving formulation (optimized formulation F-6) was subjected to stability studies at 40oc± 2oc/75% RH ±5 % for 90 days. The product was evaluated for appearance and hardness every 30 days. Drug excipient compatibility, drug content and drug release studies were conducted as per the planned scheduled.
Conclusion
Optimization of drug resin cross linking is done with parameters 1:4 as drug resin concentration ratio, stirring time 6hrs which showed 96.06% drug loading on to resin. Direct Compression method was selected to make the formulation. The granules so obtained were having good flow properties and tablets had shown satisfactory results with respect to physical parameters like hardness and friability.
Disintegration results shown that the tablets containing SSG had much more effect on disintegration with respect to concentration, where as CP also shown good disintegration ability with in the time limit but lesser effect compared to SSG. Dissolution results showed that tablets with good disintegration showed much better release than the other formulation used for the same purpose. Where as with the same weight gain as that of high resin: drug concentration cross linked tablets showed the release 99.98% in 0.1NHCL.Among mouth dissolving formulation F6, was suited formulations having best drug release in 0.1NHCL. The results obtained were shown almost above 80% of drug release in 30 minutes. As per dissolution profile F6 was concluded as best formulation.
The result obtained were compared with that of the marketed product and showed good correlation. Stability studies of the optimized best formulation conducted tablets were by keeping the tablet accelerated at 40oC ±2oC/ 75% RH ±5% for 90 days. All the parameters were within the limits after 90 days. Indicating the stability of the formulation.
Investigation of in vitro results confirmed that the formulation FVI with taste masking, good release, and stability eases to develop a suitable mouth dissolving formulation. Thus it can be concluded that stable taste masked mouth dissolving formulation can be prepared. By Using a combination of super disintegrates (SSG, CP)
References
- Leon Lachamnn; Herbert A.Liebermann; Joseph L.Kanig. The Theory and practice of Industrial Pharmacy; Third Edition; pg.no.293.
- V.Allen, Nicholas .G.Popovich, Howard.C.Ansel; Ansel’s pharmaceutical dosage form and drug delivery system 8th edition.
- Bentley’s Text book of Pharmaceutics; Eight edition; pg.no.140
- AnselLloyd;V.Allen;Jr.Nicolas;G.Popovich Pharmaceutical Dosage forms and Delivery systems;pg.no.209.
- US Food and Drug Administration, CDER Data Standards Manual, 2003, http:// http://www.fda.gov/cder/dsm/DRG/drg00201.htm accessed March 19, 2005.
- European Directorate for the Quality of Medicines, Pharmeuropa, 10 (4), 547 (1988), http:// http://www.pheur.org/, accessed March 19, 2005.
- K. Ghosh et al., “Quick Dissolving Oral Dosage Forms: Scientific and Regulatory Considerations from a Clinical Pharmacology and Biopharmaceuticals Perspective,” in Drug Delivery to the Oral Cavity: Molecules to Market, T.K. Ghosh and W.R. Pfister, Eds. (CRC Press, New York, NY, 2005), pp. 337–356.
- Dobetti, “Fast-Melting Tablets: Developments and Technologies,” Pharm. Technol. 25 (9 Suppl), 44–50 (2001).
- I. Pather, R. Khankari, and J. Siebert, “Quick-Dissolving Intraoral Tablets,” in Drug Delivery to the Oral Cavity: Molecules to Market, T.K. Ghosh and W.R. Pfister, Eds. (CRC Press, New York, NY, 2005), pp. 291–336
- Deepak, “Orally Disintegrating Tablets,” Tablets and Capsules 7 , 30–35 (2004).
- Brown, “Orally Disintegrating Tablets: Taste Over Speed,” Drug Deliv. Technol. 3 (6), 58–61 (2001).
- Brown, “Orally Disintegrating Tablets: Taste Over Speed,” Drug Deliv. Technol. 3 (6), 58–61 (2001)
- Aurora and V. Pathak, “Oral Disintegrating Technologies: Oral Disintegrating Dosage Forms: An Overview,” Drug Deliv. Technol. 5 (3), 50–54 (2005).
- C. DeRoche et al., “Consumer Preference for Orally Disintegrating Tablets Over Conventional Forms of Medication: Evolving Methodology for Medication Intake in Dysphagia,” lecture presented at the 12th Annual Meeting of the Dysphagia Research Society, San Francisco, CA, Oct. 2–4, 2003.
- Sharma et al., “Manufacturing Technology Choices for Mouth Dissolving Tablets,” Pharm. Technol. 27 (10 Suppl), 10–13 (2003).
- Wehling et al.,”Pediatric Effervescent Dosage Form,” US Patent 5,223,264 (1993).
- Wehling et al., “Effervescent Dosage Form With Microparticles,” US Patent 5,178,878 (1993).
- Wehling and S. Schuehle, “Base Coated Acid Particles and Effervescent Formulation Incorporating Same,” US Patent 5,503,846 (1996).
- V. Sastry and J. Nyshasham, “Process Development and Scale-Up of Oral Fast-Dissolving Tablets,” in Drug Delivery to the Oral Cavity: Molecules to Market, T.K. Ghosh and W.R. Pfister, Eds. (CRC Press, New York, NY, 2005), pp. 311–336.
- C. Iles et al., “Freeze-Dried Dosage Forms and Methods for Preparing the Same,” US Patent 5,188,825 (1993).







