Manuscript accepted on :August 08, 2009
Published online on: 17-11-2015
Plagiarism Check: Yes
Rohini kiran kunta3 and Varahalarao Vadlapudi1,2*
¹Phytochemical lab, Department of Botany, Andhra University, Visakhapatnam India. ²Department of Biochemistry, Dr Lankapalli bullyya Post-graduate College, Visakhapatnam - 530 013 India. ³Department of Biotechnology, Dr Lankapalli bullyya Post-graduate College, Visakhapatnam - 530 013 India. Corresponding Author E-mail:vadlapudivr@gmail.com
Abstract
In this present study as selected plant Centella asiatica belonging to the family plant Apiaceae were dried and extracted with ethanol and water using the soxhlet extraction apparatus. The antimicrobial activities of the plant extracts on the various test microorganisms, including multiple antibiotic resistant bacteria, were investigated. Antimicrobial activities of the extracts were determined by the well diffusion method.
Keywords
Antibiotic resistant bacteria; antimicrobial activity
Download this article as:| Copy the following to cite this article: Kunta R. K, Vadlapudi V. In Vitro Antibacterial Potentiality of Centella Asiatica. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Kunta R. K, Vadlapudi V. In Vitro Antibacterial Potentiality of Centella Asiatica. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=806 |
Introduction
Synthetic drugs are not only expensive and inadequate for the treatment of diseases but are also often with adulterations and side effects. Decreased efficacy and resistance of pathogens to antibiotics has necessitated development of new alternatives (Smith et al. 1994)1.Besides small molecules from medicinal chemistry, natural products are still major sources of innovative therapeutic agents for various conditions, including infectious diseases (Clardy, and Walsh. 2004) 2. Higher plants have been shown to be a potential source for new anti-microbial agents (Mitscher et al., 1987)3. Aromatic and medicinal plants are known to produce certain bioactive molecules which react with other organisms in the environment, inhibiting bacterial antimicrobial activity (Chopra et al., 1992; Bruneton, 1995)4, 5. The screening of plant extracts has been of great interest to scientist for the discovery of new drugs effective in the treatment of several diseases (Dimayuga and Garcia, 1991)6. Therefore, there is a need to search for new infection-fighting strategies to control microbial infections in the present work an attempt has been made to carry out screening for the preliminary antimicrobial activity of the medicinal plants used in Indian folk medicine for centuries which may be useful in developing lead compound to combat deadly diseases.
Centella asiatica is a small herbaceous annual plant of the family Mackinlayaceae or subfamily Mackinlayoideae of family Apiaceae, and is native to India, Sri Lanka, northern Australia, Indonesia, Iran, Malaysia, Melanesia, New Guinea, and other parts of Asia. Common names include Gotu Kola, Asiatic Pennywort, Indian Pennywort, Luei Gong Gen, Takip-kohol, Antanan, Pegagan, Pegaga, vallaarai Kula kud, Bai Bua Bok and Brahmi. In India famous as “Brahmi” used for improving the mental ability was carried out at Dr.A.Lakshmipathy Research Centre (now under CCRAS)VHS, Adyar, Chennai by (Apparao et al., 1973 )7 .
The goal of this study was to increase the knowledge of the bioactivity of parts of C.asiatia.
Materials and Methods
Plant and extraction: C. asiatia belongs to the family mackinlayaceae or subfamily mackinlayoideae of family apiaceae was taxonomically identified and the Voucher specimen is stored. The plant material were dried under shade with occasional shifting and then powdered with a mechanical grinder and stored in an airtight container. The powder obtained was subjected to successive soxhlet extraction with the organic solvents with increasing order of polarity.
Test microorganisms
Bacteria E.coli, Entetobacter aerogens, Kleibsella pneumonea, Mycobacterium glutamicus, Proteus vulgaris were obtained from Microbial Type Culture Collection (MTCC), IMTECH, Chandigarh were used as test organisms. The strains are maintained and tested on Nutrient Agar (NA) for bacteria .Active cultures were generated by inoculating a loop full of culture in separate 100ml nutrient broths and incubating on a shaker at 37oC overnight. The cells were harvested by centrifuging at 4000 rpm for 5 min, washed with normal saline, spun at 4000 rpm for 5 min again and diluted in normal saline to obtain 5 x 105cfu /mL.
Determination of antibacterial activity: The crude extracts of the different plant parts of different species were subjected to antimicrobial assay using the agar well diffusion method of (Murray, 1995)8 modified by (Olurinola., 1996)9 .
20 ml of nutrient agar was dispensed into sterile universal bottles these were then inoculated with 0.2 ml of cultures mixed gently and poured into sterile petri dishes. After setting a number 3-cup borer (6mm) diameter was properly sterilized by flaming and used to make three to five uniform cups/wells in each Petri dish. A drop of molten nutrient agar was used to seal the base of each cup.
The cups/wells were filled with 50µℓ of the extract concentration of 100mg/ml and allow diffusing for 45 minutes. The solvents used for reconstituting the extracts were similarly analyzed. The plates were incubated at 37°c for 24 hours for bacteria. The zones of inhibition were measured with antibiotic zone scale in mm and the experiment was carried out in duplicates.
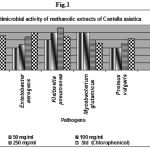 |
Figure 1:
|
Results
Table 1: Antimicrobial activity of methanol extracts of Centella asiatica.
| Bacteria | 50 mg/ml | 100 mg/ml | 250 mg/ml | Std (Chlorophenicol) |
| E.coli | 20 | 22 | 26 | 23 |
| Entetobacter aerogens | 15 | 17 | 22 | 25 |
| Kleibsella pneumonea | 20 | 25 | 28 | 22 |
| Mycobacterium glutamicus | 25 | 25 | 20 | 18 |
| Proteus vulgaris | 15 | 15 | 18 | 21 |
Volume per well: 50µl; Borer size used: 6mm;
Extract concentrations in 50, 100 and 250 mg/ml DMSO
Table 1 summarizes the antimicrobial activities of zone of inhibition of methanol (15 to 25 mm) with 50 mg/ml. The Standard (Chlorophenicol) values varying (18 to 25 mm).
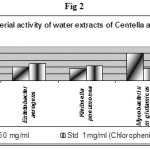 |
Figure 2:
|
Table 2: Antimicrobial activity of water extracts of Centella asiatica.
| Bacteria | 250 mg/ml | Std 1mg/ml
(Chlorophenicol) |
| E.coli | 28 | 23 |
| Entetobacter aerogens | 20 | 25 |
| Kleibsella pneumonea | 18 | 22 |
| Mycobacterium glutamicus | 40 | 18 |
| Proteus vulgaris | 20 | 21 |
Volume per well: 50µl; Borer size used: 6mm;
Extract concentration 250 mg/ml DMSO
Table 2 summarizes the antimicrobial activities of zone of inhibition of water (18 to 40 mm) with 250 mg/ml. The Standard (Chlorophenicol) values varying (18 to 25 mm).
The variation of antimicrobial activity of our extracts might be due to distribution of antimicrobial substances, which varied from fraction to fraction of the crude extract.
Discussion
The Methanolic extracts (Table 1 and fig 1) showed highest inhibition against M. glutamicus 25 (mm), followed by K. pneumonea and E.coli and lowest against E. aerogens and P. vulgaris with 50 mg/ml DMSO concentration.
The water extracts (Table 2 and fig 2) showed highest inhibition against M. glutamicus 40 (mm) and least against K. pneumonea 18 (mm) with 250 mg/ml DMSO concentration.
Conclusion
Overall, the present study provides enough data to show the potentiality of C. asiatica and it has a long history of use in medicine as a remedy for leprosy, where the causative was Mycobacterium; antimicrobial activity were conducted indicates the bio activity of C. asiatica on mycobacterium hence this plant extract can be used for treatment of mycobacterium. The plant extract has a superior activity over usual antibiotics. The present study was conducted to develop newer lead for better and safer chemotherapeutic agents from medicinal plants.
Further studies are needed to identify the pure component and establish the exact mechanism of action for antibacterial action of the plant extract.
References
- Smith, P. Hiney M.P. and Samuelsen O.B., Annual Review of Fish Diseases, 4: 273-313 (1994).
- Clardy J and Walsh C., Lessons from natural molecules, Nature, 432: 829-837 (2004).
- Mitscher L.A., Drake, S., Gollopudi, S.R.., Okwute, S.K., J. of Natural Products, 50: 1025-1040(1987).
- Chopra R.N. Nayer S. and Chopra I.C., Glossary of Indian Medicinal Plants, 3rd Edn. New Delhi: Councilof Scientific and Industrial Research, 7-246(1992).
- Bruneton J., Pharmacognosy, Phytochemistry, Medicinal plants. France: Lavoisiler Publishing Co, 265-380 (1995).
- Dimayuga, R.E. & Garcia, S.K. (1991)., J of Ethnopharmacology ., 31: 181-192
- Appa Rao M.V.R. Srinivas K and Koteshwar Rao T., J. Res Indian Med., 8: 9–16 (1973).
- Murray P. R., Baron E. J., Pfaller M. A., Tenover F. C., Yolken H. R., Manual of Clinical Microbiology, 6th Edition. ASM Press, Washington. DC., 15-18 (1995)
- Olurinola P. F., A laboratory manual of pharmaceutical microbiology. Idu, Abuja, Nigeria, 69-105 (1996).







