Lakhdar Sekhri* and Nedjimi
Department of Chemistry, University of Ouargla - 300 00 Algeria.
Abstract
A range of prochiral acetophenone derivatives have been converted efficiently to the corresponding (R)- alcohols with > 80% yield and good enantioselectivities (up to 70% ee). Cyclic ketones are also reduced with high levels of stereoselectivity with PMHS in the presence of TBAF and baker’s yeast, in particular 4-methylcyclohaxanone (trans: cis 84:16). High stereoselectivity is also observed in the reduction of 2-cyanobenzaldehyde and anti-imine was obtained in 71%.
Keywords
Asymmetric reduction; biocatalyst; backer’s yeast; TBAF, PMHS
Download this article as:| Copy the following to cite this article: Sekhri L, Nedjimi. A Convenient Procedure for the Asymmetric Reduction of Prochiral ketones using TetrabutylammoniumFluoride, Polymethylhydrosiloxane and Biocatalysts, Baker’s Yeast. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Sekhri L, Nedjimi. A Convenient Procedure for the Asymmetric Reduction of Prochiral ketones using TetrabutylammoniumFluoride, Polymethylhydrosiloxane and Biocatalysts, Baker’s Yeast. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=804 |
Introduction
The asymmetric reduction of prochiral ketons is one of the most important, fundamental and practical reactions for producing non-racemic chiral alcohols, from which many industrially important chemicals such as pharmaceuticals, agrochemicals, and natural products. Asymmetric transformations invariably involve the conversion of two dimensional substrate into a three dimensional product. For prochiral ketones such as acetophenone reduction shown in scheme-1, addition to the back face gives 1-phenyl alcohol with R configuration, while addition to the back face gives alcohol with S configuration.
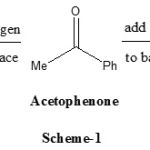 |
Scheme 1:
|
The problem, of course is that most common reducing agents, such as sodium borohydride or lithium aluminium hydride, react equally readily with either face. The most obvious solution to this problem is to use a hydride source which is itself enantiomerically pure in principal such as reagent will transfer the hydride to each face of the ketone through diastereoisomerically distinct transition state, which gives at least a fighting chance of an energy difference, and preference for addition to one face over the other.
The catalysts for the asymmetric reduction of ketones can be classified into two categories: chemical and biological methodologies. Presently, there are five chemical reagents which are extensively used in asymmetric reduction: Brown’s DIP-chloride, 1, 2 Midland’s Alpin-Borane3, Corey’s oxazaborolidines, 4,5 Nyori’s BINAL-H and BINAP-Ru complexes.6 In 1999, L. Sekhri and N. J. Lawrence7 utilized Corey’s oxazaborilidine to obtain excellent yield and enantioselectivities for a variety of diphenylphosphinoyl alcohols. The biological catalysts used for these asymmetric reductions, isolated enzymes, microbes such as yeast and fungi, and plant cell cultures have also been used. Another category of biocatalyst backer’s yeast,8-17 and vegetables,18 germinated plant19 has been applied to organic synthesis because these biocatalysts are easily obtainable from markets and easily manipulated by organic chemistry. In the other hand, Corriu and Co-workers have shown in 1980’s that esters may be reduced with PMHS by fluoride or alkoxide-iduced hydrosilylation.20 They have also published several papers describing the related potassium and cesium floride catalysed triethoxysilane reduction of esters. 21-24
Recently, N. J. Lawrence and Co-workers described the efficient reduction of esters to alcohols with polymethylhydrosiloxane (PMHS) {Me3SiO[(CH3)HSiO]nSiMe3} in the presence of titanium (IV) isopropoxide or zirconium alkoxide.25 This was followed by the description of the use of PMHS and catalytic fluoride.26 More recently, L. Sekhri and Co-workers27 extend this method to reduce several aminoacids to the corresponding aminoalcohols with polymethylhydrosiloxane, PMHS, in the presence of catalytic tetrabutylammonium fluoride, TBAF.
We now report that the same transformation can be achieved but this time with PMHS and catalytic fluoride and biocatalyst baker’s yeast.
Results and Discussion
The strategy we have adopted for this asymmetric reduction consists of the following steps:
It was, therefore decided to test whether the proketones could undergo the asymmetric reduction with baker’s yeast in the absence of TBAF and PMHS. Therefore the reaction was unsuccessful and unchanged ketone was recovered (100%).
The reaction was carried out on the same scale using TBAF and PMHS in the absence of baker’s yeast; the prochiral ketones were reduced in less than 1 min. with racemisation.
The reaction was repeated again on the same scale but using this time baker’s yeast with TBAF and PMHS, the reaction was completed after 3 min. (by t l c, silica, hexane/ diethyl ether, 5:1 disappearance of ketone). High enantioselectivity is observed in the reduction of several aromatic ketones, in particular nitro, chloro, fluoro, methylacetophenones.
This can be interpreted, in terms of the necessity of hydrogen sources such as TBAF and PMHS to perform the asymmetric reduction.
Our protocol has several advantages since PMHS is cheap and baker’s yeast is easily obtainable from markets and easily manipulated by organic chemistry. The protocol is an efficient method for the convenient reduction of prochiral aromatic ketones such as 1a, 1b, 1c, 1d and 1e to the corresponding (R)- alcohols with > 80% yield and good enantioselectivities (up to 70% ee) (Scheme-2).
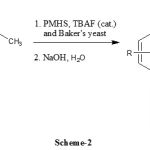 |
Scheme 2:
|
Cyclic ketones are also reduced with high levels of stereoselectivity with PMHS in the presence of TBAF and baker’s yeast, in particular 4-methylcyclohaxanone (trans: cis 84:16) (Scheme-3).
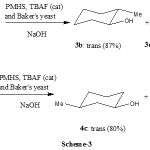 |
Scheme 3:
|
High stereoselectivity is also observed in the reduction of 2-cyanobenzaldehyde and anti-imine was obtained in 71% as shown in (Scheme-4).
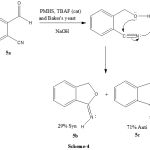 |
Scheme 4:
|
In summary, we have shown that polymethylhydrosiloxane in combination with catalytic TBAF is an excellent reducing agent for the mild reduction of aminoacids.
Experimental
All 300 MHz 1H and 75 MHz 13C NMR spectra were run on a Bruker AC 200 NMR spectrometer. Both 1HNMR 13C spectra were recorded using CHCl3 as internal standard.
Standard procedure
Three-necked round bottomed flask was fitted with magnetic stirrer bar, a reflux condenser, and an addition funnel. The flask was then charged with a mixture of prochiral ketone (1 mmol) and bread yeast (1g) tetrabutylammonium fluoride (0.02 mmol) in dry tetrahydrofuran (10 ml). The remaining neck was sealed with a septum and nitrogen line attached. A solution of polymthylhydrosiloxane (1.5 mmol) in 10 ml of THF was poured into the addition funnel and added dropwise over 30 min resulting in vigorous evolution of hydrogen. After addition of polymthylhydrosiloxane was completed and gas evolution had ceased, the reaction was completed after 3 min. (by t l c, silica, hexane/ diethyl ether, 5:1 disappearance of ketone). The mixture was stirred for further one hour and 3N NaOH (10 ml) was added cautiously until the mixture became clear. After stirring for 4 hours, the combined organic solution was passed through a short pad of Fluorisil, and the THF was removed by rotavapor and the remaining solution extracted with (3x20ml) ether. The combined organic extracts were washed with water, dried (MgSO4) and evaporated in vacuo. The residue was purified by chromatography on silica gel (pet. Ether 40-60°C/diethyl ether, 4: 2) or distillation if necessary. Typical pure yield after purification is (54%). The alcohols 2a, 2b, 2c and 2d are identified by the following spectroscopic data:
p-Phenylethanol 2a
(83% yield); 1H (CDCl3, 300 MHz): δ (ppm) : 1.4 (3H, d, CH3CHOH-), 3.0 (1H, br. s,OH), 4.8 (1H, q, -CHOH), 7.1-7.3 (5H, m, Ar-H).
Chlorophenylethanol 2b
(96% yield); 1H (CDCl3, 300 MHz): δ (ppm): 1.3 (3H, d, CH3CHOH-), 3.5 (1H, br.s, OH), 4.7 (1H, q, -CHOH), 7.0-7.3 (4H, m, Ar-H); 13C (CDCl3, 75MHz): δ (ppm) = 28.03 (CH3CHOH), 69.54 (-CHOH), 126.93 (CH, Ar), 128.25 (CH, Ar), 132.94 (C, Ar), 144.44 (C, Ar).
Nitrorophenylethanol 2c
(90% yield); 1H (CDCl3, 300 MHz): δ (ppm): 1.4 (3H, d, CH3CHOH-), 2.6 (1H, br.s, OH), 4.9 (1H, q, -CHOH), 7.4 (2H, d, Ar-H), 8.1 (2H, d, Ar-H); 13C (CDCl3, 75MHz): δ (ppm) = 25.27 (CH3CHOH), 69.27 (-CHOH), 123.56 (CH, Ar), 126.04 (CH, Ar), 146.86 (C, Ar), 153.28 (C, Ar).
p-Tolylethanol 2d
(92% yield).
1H (CDCl3, 300 MHz): δ (ppm) : 1.3 (3H, d, CH3CHOH-), 2.3 (3H, s, 4-CH3C6H4-), 3.0 (1H, br. s, OH), 4.7 (1H, q, -CHOH), 7.0-7.2 (4H, m, Ar-H; 13C (CDCl3, 75MHz): δ (ppm) = 21.22 (CH3CHOH-), 25.21 (4-CH3C6H4-), 20.25 (-CHOH), 125.51 (CH, Ar), 129.25 (CH, Ar), 137.15 (C, Ar).
Fluorophenylethanol 2e
(86% yield); 1H (CDCl3, 300 MHz): δ (ppm): 1.4 (3H, d, CH3CHOH-), 3.2 (1H, br.s, OH), 4.8 (1H, q, -CHOH), 6.8-7.0 (2H, m, Ar-H), 7.1-7.3 (2H, m, Ar-H).
Trans-2-methylcyclohexanol 3b
(84% yield, 87% trans); 1H (CDCl3, 300 MHz): δ (ppm): 0.9 (3H, d, J= 6.4Hz, CH3), 1.2 (4H, m, –CH2CH2-), 1.6 (1H, m, -CHCH3), 1.7 (2H, m, -CH2-), 2.6 (1H, br.s, OH), 3.2 (1H, m, -CHOH); 13C (CDCl3, 75MHz): δ (ppm) = 16.70 (CH3), 23.18 (CH2), 25.27 (CH2), 26.36 (CH2), 30.20 (CH2), 39.90 (-CHCH3), 76.63 (-CHOH).
Cis-2-methylcyclohexanol 3c
(84% yield, 13% cis); 1H (CDCl3, 300 MHz): δ (ppm): 0.8 (3H, d, J= 6.4Hz, CH3), 1.0 (4H, m, –CH2CH2-), 1.5 (2H, m, -CH2-), 2.3 (1H, m, -CHCH3), 2.6 (1H, br.s, OH), 3.9 (1H, m, -CHOH); 13C (CDCl3, 75MHz): δ (ppm) = 13.99 (CH3), 20.71 (CH2), 24.12 (CH2), 25.54 (CH2), 28.67 (CH2), 38.41 (-CHCH3), 70.94 (-CHOH).
Trans-3-methylcyclohexanol 4b
(84% yield, 80% trans); 1H (CDCl3, 300 MHz): δ (ppm): 0.9 (3H, d, J= 6.4Hz, CH3), 1.0-1.6 (6H, m, –CH2CH2CH2-), 1.8 (1H, m, -CHCH3), 2.2 (1H, br.s, OH), 3.0 (1H, m, -CHOH); 13C (CDCl3, 75MHz): δ (ppm) = 18.97 (CH3), 24.27 (CH2), 25.27 (CH2), 26.16 (CH2), 28.86 (CH2), 33.44 (-CHCH3), 70.89 (-CHOH).
Cis-3-methylcyclohexanol 4c
(84% yield, 20% cis); 1H (CDCl3, 300 MHz): δ (ppm): 0.8 (3H, d, J= 6.4Hz, CH3), 1.1-1.6 (6H, m, –CH2CH2CH2-), 2.3 (1H, m, -CHCH3), 3.2 (1H, br.s, OH), 3.6 (1H, m, -CHOH); 13C (CDCl3, 75MHz): δ (ppm) = 16.64 (CH3), 20.80 (CH2), 25.29 (CH2), 28.68 (CH2), 30.22 (CH2), 33.66 (-CHCH3), 76.38 (-CHOH).
Imine (anti) 4b
85% yield (71% anti); 1H (CDCl3, 300 MHz): δ (ppm): 4.7 (2H, s, CH2), 6.4 (1H, br.s, NH), 7.1-7.4 (3H, m, Ar-H), 7.5 (1H, d, Ar-H); 13C (CDCl3, 75MHz): δ (ppm) = 61.45 (CH2), 109.88 (C), 121.55 (CH), 127.10 (CH), 128.45 (CH), 133.12 (CH), 143.90 (C), 169.23 (CN); M/Z (FAB): 134 [(M+H),+100], 129 (25), 119 (20), 116 (35), 107 (20), 97 (18), 93 (20), 97 (18), 93 (20), 91 (63), 89 (18).
Imine (syn) 4c
85% yield (29% syn); 1H (CDCl3, 300 MHz): δ (ppm): 5.1 (2H, s, CH2), 6.4 (1H, br.s, NH), 7.1-7.4 (3H, m, Ar-H), 7.7 (1H, d, Ar-H); 13C (CDCl3, 75MHz): δ (ppm) = 71.62 (CH2), 117.37 (C), 123.27 (CH), 127.55 (CH), 132.75 (CH), 133.91 (CH), 145.82 (C), 169.23 (CN).
Acknowledgements
We thank Prof. R. E. Banks, Dr. A. Brisdon, Dr. N. J. Lawrence and the staff of analytical and spectroscopic services of the Chemistry Department, University of Manchester (UMIST), U.K. for their assistance. We thank also Mr. Chakroud Djamel, Mr. Maiza Abderrazak, Mr. Maaouche Abderrazak, Ph.D student at Manchester University, and Dr. Derouiche Laid for their assistance.
References
- C. Brown, W. S. Park, B. T. Cho, P.V. Ramachandran, J. Org. Chem., 52, 5406 (1987).
- M. Coppola, H. F. Schuster, Asymmetric Synthesis, Construction of chorale molecules using aminoacids, Wiley-interesc, Publ., Chapter 1, 2 and 3 (1987).
- M. Midland, Chem. Rev., 89, 1553 (1989).
- J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc., 109, 5553 (1987).
- J. Corey, R. K. Bakshi, S. Shibata, V. K. Singh, J. Am. Chem. Soc., 109, 7925 (1987).
- K. Henri, T. Maurice, C. F. Jean, Tetrahedron Lett., 42, 4959 (1991).
- Sekhri, N. J. Lawrence, J. Soc. Alger.Chim., 10, 9 (2000).
- Yasohara, N. Kizaki, J. Hasegawa, S. Takahashi, M. Wada, M. Katoka, S. Shimizu, Appl. Microbial. Biotechnol. 51, 847 (1999).
- Wada, M. Katoka, H. Kawabata, Y. Yasohara, N. Kizaki, J. Hassegawa, S. Shimizu, Biosc. Biotech. Biochem. 62, 280 (1999).
- A. Levene, A. Walti, Org. Synth., 2, 545 (1943).
- Barry, H. B. Kagan, Synthesis, 453 (1991).
- Ramaswamy, A. C. Oehischlager, Tetrahedron, 47, 1145 (1991).
- Ferraboschi, P. Grisenti, A. Manzocchi, E. Santaniello, J. Chem. Soc., Perkin, 1, 2469 (1990).
- Sato, Y. Okumera, J. Italai, T. Fujisawa, Chem. Lett. 1537 (1988).
- Iriuchijimma, N. Kojima, Agric. Biol. Chem. 22, 451 (1978).
- Nakamura, Tetrahedron: Asymmetry, 14, 2659 (2003).
- Nakamura, R. Yamanaka, T. Matsuda, T. Harada, Tetrahedron, Asymmetry, 2003, 14, 2659-2681.
- Utsukihara, S. Watanabe, A. Tomiyama, W. Chai, C. A. Horiuchi, J. Mol. Catal. B: Enzyme, 2006, 41, 103-109.
- Kiyoko Matsuo, Sei-ichiro Kawabe, Yosuke Tokuda, Takashi Eguchi, Rio Yamanaka and Kaoru Nakamura, Tetrahedron: Asymmetry, 19, 157 (2008).
- Chuit, R. J. P. Corriu, R. Perz, C. Reyé, Synthesis, 981 (1982).
- Boyer, R. J. P. Corriu, R. Perz, C. Reyé, Tetrahedron, 37, 2165 (1981).
- Boyer, R. J. P. Corriu, R. Perz, M. Poirier, C.Reyé, Synthesis, 558 (1981).
- Boyer, R. J. P. Corriu, R. Perz, C. Reyé, J. Chem. Soc., Chem. Commun, 121 (1981).
- Boyer, R. J. P. Corriu, R. Perz, C. Reyé, Tetrahedron, 39, 999 (1983).
- Drew, N. J. Lawrence, F. David, L. Sekhri, Synlett, 989 (1997).
- Drew, N. J. Lawrence, Abstracts of papers of the Am. Chem. Soc., 212 (1996).
- Sekhri, M. Drid, Oriental. J. Chem., 23 (3), 869 (2007).







