Kadambri Gupta, Anupriya Sachar, Krishma Gupta and Sheetu Raina
Department of Zoology, University of Jammu, Jammu, Jammu and Kashmir - 180 006 India.
Abstract
This paper reports the histopathological analysis of various organs of minor carp, Labeo boga (Ham.) following exposure to sublethal concentration of lindane (gamma-hexachloro-cyclohexane) of 1.05mg/l. Cellular vacuolization, distended sinusoids and hepatocyte swelling were the conspicuous changes observed in liver tissue which resulted in loss of normal structure and function. Various abnormalities observed in the splenic tissue included vacuolization, deposition of haemosiderin pigments and proliferation of melanomacrophage centres. Kidney too demonstrates various degenerative changes. The results of this histological analysis of various fish tissues indicate a direct co-relation between insecticide exposure and histopathological disorders observed.
Keywords
Lindane; Haemosiderin; Melanomacrophage centers
Download this article as:| Copy the following to cite this article: Gupta K, Sachar A, Gupta K, Raina S. Effect of Lindane on the Haemopoietic Tissues in a Minor Carp Labeo Boga (Ham.). Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Gupta K, Sachar A, Gupta K, Raina S. Effect of Lindane on the Haemopoietic Tissues in a Minor Carp Labeo Boga (Ham.). Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=800 |
Introduction
Lindane is an organochloride insecticide used as a broad spectrum insecticide which controls insects of wide variety of food crops including fruits as well as cereals, maize and other grains by contact action. Other than targeted species, these insecticides from agricultural runoff affect even all non-target organisms inhabiting aquatic environment (Burkepile et al., 2000). Insecticides at high concentrations are known to reduce the survival, growth and reproduction of these biotic organisms including fish (Rahman et al., 2002). Due to their residual effects, these insecticides can badly damage vital organs including liver, kidney and spleen. To determine the extent of the impairment, histopathological studies need to be conducted because they have been prove to be a sensitive tool to detect direct effects of chemical compounds within target organs of fish in laboratory experiments (Schwaiger et al., 1996).
During present studies the histopathological effects of sublethal concentration of lindane (found to be 1.05mg/l after LC50 experiments of 96hrs. duration) on the haemopoietic tissues viz., liver, kidney and spleen of minor carp, Labeo boga (Ham.) have been studied with main objective to find out the extent of the lethality.
Materials and Methods
The fish were dissected open in ringer saline solution and then liver, anterior kidney and spleen were fixed in bouin’s fixative. After post-fixation treatment and routine dehydration and clearing, tissues were embedded in histowax of 54-56ºc temperature. 5-7µm thick section cut on microtome and was stained using haematoxylin-eosin (Bancroft and Stevens, 1977).
Various pathological incidences were scanned and photographed with Axio Imager Al (Carl Zeiss) microscope.
Results and Discussions
Liver
The normal histology of liver and its histopathology of L.boga and is depicted in Table (1) and Fig. (1-4) as under:-
Table 1:
| S.No. | Liver (control) | Liver (lindane treatment) |
| 1 | Presence of hepatocytes | Necrosis of hepatocytes |
| 2 | Presence of Sinusoids | Widening or distention of sinusoids and vacuolization of the tissue |
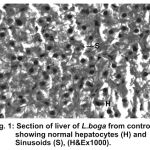 |
Figure 1:
|
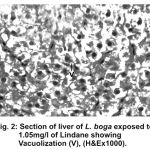 |
Figure 2:
|
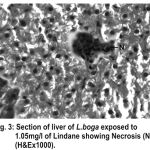 |
Figure 3:
|
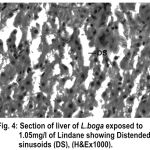 |
Figure 4:
|
Liver has the ability to degrade toxic compounds taken either from outside along with food or these produced within but its regulatory mechanisms can be overwhelmed if concentration of these compounds exceed the tolerable limit and hence may subsequently result damaging its cellular function (Brusle et al., 1996). In the present study, liver exhibited necrosis, vacuolation and distention of blood sinusoids (Fig. 2, 3 & 4). Similar to our findings Choudhary et al (2003) found that organochloride led to hepatic injury when fish were exposed to sublethal concentrations of endosulfan. Similar observations have been made for the white fish (Coregonus clupeaformis) exposed to nickel (Ptashynski et al., 2001), Salvelinus namayaish exposed to lindane (Gill et al., 1988), Oreochromis niloticus exposed to malathion (Elazaby et al., 2001), Labeo rohita exposed to carbofuran and cypermethrin (Sarkar et al., 2005) and Cirrhina mrigala exposed to fenvelarate (Velmurugan et al., 2007).
Necrosis of the hepatocytes and distention of blood sinusoids in the lindane treated fish implies that haemopoiesis being one of the synthetic metabolic process possibly get hampered by the insecticide intoxication. In this context the observation made by Gingerich (1982) supporting that the randomly distributed vacuoles commonly observed in the hepatocytes of affected animals indicate an imbalance between the rate of synthesis of substance in the parechymal cells and their release into the circulation. Present finding in L.boga simply authenticate that organochlorides (lindane used presently) does hamper the normal physiology of liver and hence the haemopoiesis too which is one of the most important function it perform in fishes.
Kidney
Compared to the normal histological details (table 2) of kidney showing kidney tubules (Fig.5) and haemopoietic tissue (Fig.5). Cellular structure of kidney exposed to lindane (fig. 6-8) showed changes viz.,
| S.No. | Kidney (control) | Kidney (lindane treatment) |
| 1 | Presence of haemopoietic tissue | Necrosis of haemopoietic tissue |
| 2 | Presence of kidney tubules | Degenerated kidney tubules and tubular vacuolization |
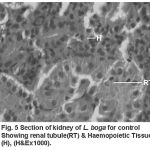 |
Figure 5:
|
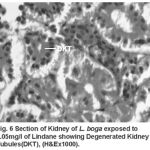 |
Figure 6:
|
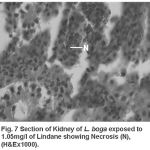 |
Figure 7:
|
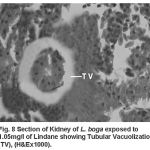 |
Figure 8:
|
Degenerated kidney tubules (Fig. 6)
Necrosis of haemopoietic tissue (Fig. 7)
Tubular vacuolization (Fig. 8)
Present results of histopathological changes following exposure to organochloride are in conformity to those of Gupta and Dalela (1987), Tripathi and Shukla (1990), Hart et al. (1997), Dutta et al. (1997), Das and Mukherjee (2000), Rahman et al. (2002), Sarker et al. (2005), Velmurugan et al. (2007), Gupta (2008) in respective fishes they studied. Necrosis of the haemopoietic tissue appear to be the causative for decline in the haematological and leucocyte percentage values (Bucher and Hofer, 1993, Ranzani-Paiva et al., 1997).
As an important organ on the immunity response elaboration (Zapata and Cooper, 1990) pathological changes induced in kidney tissue present author suggest that by affecting defense system, these changes disturbs the animal’s homeostasis and health.
Spleen
Yet another component of the haemopoietic system in fishes. Upon treatment with lindane disrupts its normal histology and results in various histopathological effects which are depicted in table (3 ) and fig (9-12 )
| S.No. | Liver (control) | Liver (lindane treatment) |
| 1 | Presence of hepatocytes | Necrosis of hepatocytes |
| 2 | Presence of Sinusoids | Widening or distention of sinusoids and vacuolization of the tissue |
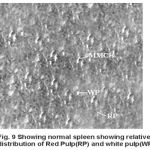 |
Figure 9:
|
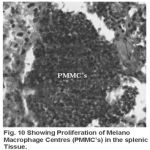 |
Figure 10:
|
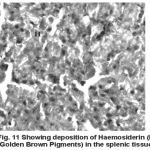 |
Figure 11:
|
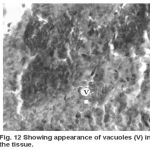 |
Figure 12:
|
Melanomacrophage centers (MMC’s) in the spleen forms an integral component of reticuloendothelial system that acts as main repository for iron containing compounds needed for erythropoiesis (Agius, 1979, Zapata et al. 1996). Proliferation of MMC’s in the spleen as observed presently has been associated with either normal ageing or due to prolonged starvation or infectious diseases (Reviewed by Couillard et al., 1999). Deposition of haemosiderin pigments (which is one of the breakdown products of haemoglobin from senescent erythrocytes, Zapata and Cooper, 1990) causing the disease haemosiderosis. Vacuolization of tissue affects the rate of synthesis of substances in the tissue and their subsequent release.
In view of the above, histopathological alterations in L.boga results in malfunctioning of the splenic tissue thereby affecting immune system of the fish.
Conclusion
In view of the deleterious effects lindane produced even at low concentration (sublethal concentration), it is therefore recommended that.
The effluents containing insecticides should be disposed after their proper treatment and
There should be strict and regular monitoring of these toxicants in the water bodies to check possible environmental hazards. It is all the more important because not only haemopoietic organ (which have been studied presently) but the overall fish quality get impaired and the impact on human being are often overwhelmed.
Bibliography
Refrencess
- Agius, C. (1979). The role of melano-macrophage centers in iron stage in normal or diseased fish. Journal of Fish Diseases. 2: 337-343.
- Bancroft, J.D. and Stevens, A. (1977). Theory and practice of histological techniques. Churchill Livingstone.
- Brusle, J., Gonzalez, I. and Anadon, G. (1996). The structure and function of fish liver. In: Fish Morphology (Eds. J.S.D. Munshi and H.M. Dutta), Science Publishers Inc., New York.
- Bucher, F. and Hofer, R. (1993). Histological and enjyme histochemical changes in the kidney of male bullhead (Cotus gobio) during the spawning period. Fish Biol., 42: 403-409.
- Burkepile, D., Vethaak, D., Lang, T. and Mellergard, S. (1996). Common diseases and parasites of fish in the North Atlantic., Training Guide for identification. International council for the exploration of the sea techniques in Marine Environmental Sciences, Copenhagen.
- Choudhary, N., Sharma, M., Verma, P. and Joshi, S.C. (2003). Hepato and nephrotoxicity in rat exposed to endosulfan. Env Biol., 24: 305-8.
- Couillard, C. M., Williams, P.J., Courtenay, S.C. and Rawn, G.P. (1999). Histopathological evaluation of Atlantic tomcod (Microgadus tomcod) collected at estuarine sites receiving pulp and pulp mill effluent. Aquatic Toxicology, 44: 263-278.
- Das, B.K. and Mukherjee, S.C. (2000). Sublethal effect of quinalphos on selected blood parameters of Labeo rohita (Ham.) fingerlings. Asian Fish Science, 13: 225-233.
- Dutta, H.M., Qadri, N., Ojha, J., Singh, N.K., Ahikari, S., Munshi, J.S.D. and Roy, P.K. (1997). Effect of diazinon on macrophages of blue gill fish (Lapomis machrochirus), a cytochemical evaluation. Environ. Contam. Toxicol., 58: 135-141.
- Elezaby, M.M., El-Serafy, S., Heckmann, R., Sharf Eldeen, K.H. and Sessek, M.M. (2001). Effect of some toxicants on the fresh water fish Oreochromis J. Egypt Ger. Soc. Zool., 36:407-434.
- Gill, T.S., Pant, J.C. and Pant, J. (1988). Gill, liver and kidney lesions associated with experimental exposures to carbaryl and dimethoate in the fish (Puntius conchonius, Ham.). Bull Environ Contam Toxicol., 41: 71-8.
- Gingerich, W.H. (1982). Hepatic toxicology of fishes. In “Aquatic Toxicology” (Ed. Weber L.) Raven Press, New York: 55-105.
- Gupta, A.K. and Dalela, R.C. (1987). Kidney damage in Notopterus notopterus (Pallus) following exposure to phenolic compounds. Environ. Biol., 8: 167-172.
- Gupta, K. (2008). Copper toxicity induced changes in haematological and haemopoietic tissues in Puntius sophore (Ham.). M.Phil Dissertation, University of Jammu, Jammu.
- Hart, L.J., Smith, S.A., Smith, B.J., Robertson, J. and Holladay, S.D. (1997). Exposure of tilapian fish to the pesticide lindane results in hypocellularity of the primary haematopoietic organ (pronephros) and spleen without altering activity of phagocytic cells in these organs. Toxicology, 118: 211-221.
- Ptashynski, M.D., Pedlar, R.M., Evans, R.E., Baron, C.L. and Klaverkamp, J.F. (2001). Toxicology of dietary nickel in lake white fish (Coregonus clupeaformis). Toxicol., 58: 229-47.
- Rahman, M.Z., Hossain, Z., Mollah, M.F.A. and Ahmed, G.U. (2002). Effect of diazinon 60EC on Anabas testudineus, Channa punctatus and Barbodes gonionotus (Ed. M.V. Gupta). Aquacultural Section of the Network of Tropical Aquaculture and Fisheries Professionals (NTAFFP). Alluabyte, 25 (2).
- Ranzani-Paiva, M.J.T., Rodriguez, E.L., Eiras, A.C., Veiga, M.L. and Pacheco, F.J. (1997). Alteracoes hematologices em curimbata Prochilodus scorfa Steindachner 1881, expose to ao Dipterex 500 (Trichlorofon). Inst. Pesca., 24 (especial): 187-196.
- Sarkar, B., Chatterjee, A., Adhikari, S. and Ayyapan, S. (2005). Carbofuran and cypermethrin induced histopathological alterations in the liver of Labeo rohita (Hamilton) and its recovery. Journal of Applied Icthyology, 21: 131-135.
- Schwaiger, J., Fent, K., Stecher, H., Ferling, H., and Negla, R.D. (1996). Effects of sublethal concentrations of triphenyl tinacetate on rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol., 30: 327-34.
- G. and Shukla, S.P. (1990). Enzymatic and ultra structural changes in a freshwater catfish- Impact of methyl parathion. Biomed. Environ. Sci., 3: 166-182.
- Velmurugan, B., Selvanayagam, M., Cengiz, E.I. and Unlu, E. (2007). The effects of fenvalerate on different tissues of freshwater fish Cirrhina mrigala. Journal of environmental Science and Health, 42 (2): 157-163.
- Zapata, A,G., Chiba, A. and Varas, A. (1996). Cell and tissues of the immune system of the fish. In: The fish immune system. Organism. Pathogen and Environment (Ed. G.K. Iwama and T. Nakanishi). Pp. 1-62. Academic press, New York.
- Zapata, A.G. and Cooper, E.L. (1990). The immune system: Comparative Histophysiology. John Wiley and Sons, Chichester, England.







