Manuscript accepted on :July 18, 2009
Published online on: 16-11-2015
Plagiarism Check: Yes
V. Ravichandiran1, V. Suba1, B. Senthilnathan*1, K. Rajachandrika1, S. Padmapriya1, T. Saraswathy2
1Vels college of Pharmacy, Pallavaram, Chennai India.
2Lecturer,Madras Medical College, Chennai - 3 India.
Corresponding Author E-mail:sen03mpharm@gmail.com
Abstract
Pulsatile drug delivery systems (PDDS) are gaining importance as these systems deliver the drug at specific time as per the pathophysiological need of disease, resulting in improved patient compliance. Inorder to emulate innate circadian rhythms, a reasonable and generally accepted rationale is a delivery system capable of releasing drugs in pulsatile fashion rather than continuous delivery at predetermined times. PDDS are promising in diseases like asthma, peptic ulcer, arthritis, cardiovascular diseases, attention deficit syndrome in children, hypercholesterolemia. PDDS can be classified into time controlled systems and stimuli controlled systems. This article includes advantages, drawbacks, methodologies, their applications in different pathological conditions.
Keywords
PDDS-Pulsatile drug delivery systems; CR-controlled release; SR-sustained release
Download this article as:| Copy the following to cite this article: Ravichandiran V, Suba V, Senthilnathan B, Rajachandrika K, Padmapriya S, Saraswathy T. Pulsatile Drug Delivery System. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Ravichandiran V, Suba V, Senthilnathan B, Rajachandrika K, Padmapriya S, Saraswathy T. Pulsatile Drug Delivery System. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=749 |
Introduction
For several drugs or therapies, a pulsatile release profile, where the drug is released completely after a defined lag time, is advantageous [1, 2]: for drugs which develop biological tolerance, for drugs with an extensive first pass metabolism, for drugs targeted to a specific site in the intestinal tract, e.g. to the colon, protecting the drug from degradation and for the adaptation of drug needs to circadian rhythms of body functions or diseases [3,4]. Several approaches to pulsatile drug delivery exist. Most systems contain a drug reservoir, surrounded by a barrier which either erodes or dissolves [5–10], or ruptures [11–15]. With eroding or dissolving systems, a potential problem is the retardation and therefore not immediate drug release after the loss of the barrier function [16] or a premature release, seen in particular with highly water-soluble drugs.
Pulsatile drug delivery systems (PDDS) are characterized by a rapid drug release after a predetermined lag time and can be classified as single unit (e.g. tablet or capsule) or multiparticulate (e.g. pellets) systems.
Pulsatile drug delivery systems release active ingredient completely and rapidly after a defined lag time [17] Such systems are advantageous for (i) drugs with an extensive first pass metabolism and developed biological tolerance, (ii) the targeting of locally absorbed or acting drugs to a specific site in the intestinal tract (e.g. colon), (iii) the adaptation of the therapy to chronopharmacological needs [18,19]
Drugs are released in an immediate or extended fashion. However, in recent years, pulsatile drug release systems are gaining growing interest. A pulsatile drug release, where the drug is released rapidly after a well defined lag-time, could be advantageous for many drugs or therapies [20-22]. Pulsatile release systems can be classified in multiple-pulse and single-pulse systems [23]
These systems constitute a relatively new class of devices, the importance of which is especially connected with the recent advances in chronopharmacology[24]. Particular rhythms in the onset and extent of symptoms were observed in diseases such as, bronchial asthma ,myocardial infarction, angina pectoris, rheumatic disease, ulcer, diabetes, attention deficit syndrome, hypercholesterolemia, and hypertension .
Time-delayed dosage forms permit the delivery of drugs after a pre-determined lag-time [25] and this can have clinical significance where a disease state has shown circadian rhythm dependency [26]. For example, there are morning peaks in the onset of myocardial infarction, sudden death and stable angina in coronary heart disease patients [27]; provision of a suitable medication, such as verapamil [28], at the right time could potentially alleviate these conditions.
The application of pulsatile release systems can be advantageous to adapt a drug therapy to chronopharmacological needs or to target a drug to a specific site in the gastrointestinal tract (GIT), e.g., to the colon.
Methodologies For Pulsatile Drug Delivery
Methodologies for the pulsatile drug delivery system can be broadly classified into three classes
Time controlled
Stimuli induced
Externally regulated
Time controlled pulsatile release system
In time controlled drug delivery systems pulsatile release is obtained after a specific time interval in order to mimic the circadian rhythm. Such type of pulsatile drug delivery system contains two components: one is of immediate release type and other one is a pulsed release type. Various Methodologies that can be used for time controlled
Pulsatile release systems are discussed in following section.
Delivery systems with rupturable coating layer
These systems consist of an outer release controlling water insoluble but permeable coating subject to mechanically induced rupture phenomenon. Recently different systems based on hard gelatin capsules and tablet core were described, all coated by inner swellable and outer rutpurable layer. The film rupture may be attained by including swelling, osmotic or effervescent additives in the reservoir8. By optimizing the system, drug release can be obtained at specific time interval. Sungthongjeen et al developed a tablet system consisting of core coated with two layers of swelling and rupturable coatings wherein they used spray dried lactose and microcrystalline cellulose in drug core and then core was coated with swelling polymer croscarmellose sodium and an outer rupturable layer of ethylcellulose. Further Thombre et al developed osmotic drug delivery using swellable core technology wherein formulations consists of a core tablet containing the drug and a water swellable component, and one or more delivery ports with rutpurable coating layer.
Delivery systems provided with erodible coating layers
In such systems the drug release is controlled by the dissolution or erosion of the outer coat which is applied on the core containing drug. Time dependent release of the active ingredient can be obtained by optimizing the thickness of the outer coat developed an oral dosage form devised to release drugs following a programmed time period after administration based on this concept. System is composed of a drug-containing core and a hydrophilic of delaying the drug release through slow interaction with aqueous fluids. There are two types of eroding systems.They are
Bulk eroding systems
Poly(lactide-co-glycolide)PLGA is a bulk eroding polymer, where the ingress of water is faster than the rate of degradation. In this case, degradation takes place throughout the polymer sample, and proceeds until a critical molecular weight is reached, at which point degradation products become small enough to be solubilised. At this point, the structure starts to become significantly more porous and hydrated, and it is possible for drug dissolved in the polymer matrix to be released, corresponding to the time required for critical molecular weight to be reached. Hence there is a time lag before the drug can be released, corresponding to the time required for critical molecular weight to be reached.
Surface eroding systems
Certain polymers such as poly(ortho) esters and poly anhydrides, degrade by surface erosion. This means that the rate of degradation of the polymer is such that mass loss is faster than the ingress of water in to bulk. Hence the sample is eroded from the surface, at controlled and predictable rate. Drug dissolved in the polymer is released at constant rate as the erosion progresses, provided that the device surface area does not change.
Capsule shaped system provided with release controlling plug
These systems contain release controlling plug between immediate release compartment and pulsed release compartment. On contact with aqueous fluids,the cap rapidly dissolves thereby releasing the immediate release component followed by pulsed release component. The lag time is provided by the plug which is inserted in to the body. In an approach used by Jimoh et al, pulsatile release was achieved by generation of hydrostatic pressure inside the capsule. A hollow biodegradable capsule of poly(lactic acid) (PLA) containing the drug along with citric acid / sodium bicarbonate and glucose was prepared. Thin poly(lactide-co-glycolide) (PLGA) membrane (to allow water penetration inside the capsule) was utilized on one end. Water penetrates into the capsule through the thin poly(lactide-co-glycolide) (PLGA) membrane side, which generates effervescence due to reaction caused between the citric acid and sodium bicarbonate, generating carbon dioxide gas that accumulates in the capsule and finally ruptures the thin membrane.
Stimuli induced pulsatile drug delivery systems
In these systems there is release of the drug after stimulation by any biological factor like temperature, or any other chemical stimuli. These systems are further classified in to temperature induced systems and chemical stimuli induced system, on the basis of stimulus.
Thermo –responsive hydrogel systems
This system uses hydrogels that undergo reversible volume changes in response to changes in temperature .These gels shrink at a transition temperature that is related to the lower critical solution temperature(LCST) of the linear polymer from which the gel is made. Therm-sensitive hydrogels have a certain affinity for water and thus swell at temperatures below the transition temperature, where as the expel water and thus shrink at temperature above the transition temperature.
Chemical stimuli induced Pulsatile systems
Glucose-responsive insulin release devices
In case of diabetes mellitus there is rhythmic increase in the levels of glucose in the body requiring injection of the insulin at proper time. Several systems have been developed which are able to respond to changes in glucose concentration. One such system includes pH sensitive hydrogel containing glucose oxidase immobilized in the hydrogel. When glucose concentration in the blood increases glucose oxidase converts glucose into gluconic acid which changes the pH of the system. This pH change induces swelling of the polymer which results in insulin release. Insulin by virtue of its action reduces blood glucose level and consequently gluconic acid level also gets decreased and system turns to the deswelling mode thereby decreasing the insulin release. Examples of the pH sensitive polymers includes N,Ndimethylaminoethyl methacrylate, chitosan, polyol etc. Obaidat and Park prepared a copolymer of acryl amide and allyl glucose. The side chain glucose units in the copolymer were bound to concanavalin A18. These hydrogels showed a glucose-responsive, sol–gel phase transition dependent upon the external glucose concentration. Okano et al developed the system based upon the fact that boronic acid moiety forms reversible bonds with polyol compounds including glucose. They used water-soluble copolymers, containing phenylboronic acid side chains which showed formation of a reversible complex gels with polyol compounds such as poly(vinyl alcohol) (PVA)19. Such complexes dissociated after the addition of glucose in a concentration dependent manner
Inflammation-induced pulsatile release
On receiving any physical or chemical stress, such as injury, fracture etc., inflammation take place at the injured sites. During inflammation, hydroxyl radicals are produced from these inflammation-responsive cells. Yui and co-workers focused on the inflammatory induced hydroxyl radicals and designed drug delivery systems, which responded to the hydroxyl radicals and degraded in a limited manner. They used hyaluronic acid (HA) which is specifically degraded by the hyaluronidase or free radicals. Degradation of HA via the hyaluronidase is very low in a normal state of health. Degradation via hydroxyl radicals however, is usually dominant and rapid when HA is injected at inflammatory sites. Thus, it is possible to treat patients with inflammatorydiseases like rheumatoid arthritis;using anti-inflammatory drug incorporated HA gels asnew implantable drug delivery systems.
Drug release from intelligent gels responding to antibody concentration
There are numerous kinds of bioactive compounds which exist in the body. Recently, novel gels were developed which responded to the change in concentration of bioactive compounds to alter their swelling/de swelling characteristics. Special attention was given to antigen-antibody complex formation as the cross-linking units in the gel, since such interaction is very specific. Utilizing the difference in association constants between polymerized antibodies and naturally derived antibodies towards specific antigens, reversible gel swelling/deswelling and drug permeation changes occurs
pH sensitive drug delivery system
Such type of pulsatile drug delivery system contains two components one is of immediate release typea nd other one is pulsed release which releases the drug in response to change in pH. In case of pH dependent system advantage has been taken of the fact that there exists different pH environment at different parts of the gastrointestinal tract. By selecting the pH dependent polymers drug release at specific location can be obtained. Examples of pH dependent polymers includes cellulose acetate phthalate, polyacrylates, sodium carboxymethylcellulose. Thesepolymers are used as enteric coating materials so as to provide release of drug in the small intestine. Yang et al developed pH-dependent delivery system of nitrendipine in which they have mixed three kinds of pH dependent microspheres made up of acrylic resins Eudragit E-100, Hydroxypropylmethylcellulose phthalate and Hydroxypropylmethylcellulose acetate succinate as pH dependent polymers. In one of the study carried out by Mastiholimath et al attempt was made to deliver theophylline into colon by taking the advantage of the fact that colon has a lower pH value (6.8) than that of the small intestine (7.0–7.8). So, by using the mixture of the polymers, i.e. Eudragit L and Eudragit S in proper proportion, pH dependent release in the colon was obtained.
Externally regulated systems
For releasing the drug in a pulsatile manner, another way can be the externally regulated systems in which drug release is programmed by external stimuli like magnetism, ultrasound, electrical effect and irradiation. Magnetically regulated system contains magnetic beads in the implant. On application of the magnetic field, drug release occurs because of magnetic beads. Saslawski et al. developed different formulation for in vitro magnetically triggered delivery of insulin based on alginate spheres23. In case of ultrasonically modulated systems, ultrasonic waves causes the erosion of the polymeric matrix thereby modulating drug release. Miyazaki et al evaluated the effect of ultrasound (1 MHz) on the release rates of bovine insulin from ethylenevinyl alcohol copolymer matrices and reservoir-type drug delivery systems in which they found sharp drop in blood glucose levels after application of ultrasonic waves24. Also irradiation with light rays the desired drug release pattern. Mathiowitz et al developed photochemically controlled delivery systems prepared by interfacial polymerization of polyamide microcapsules. For this purpose, azo bis isobutyronitrile (AIBN), a substance that photochemically emanates nitrogen gas, was incorporated. Due to exposure of azobisisobutyronitrile to light, causing release of nitrogen and an increase in the pressure which ruptures the capsules thereby releasing the drug.
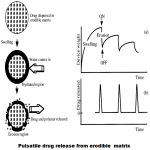 |
Figure 1:
|
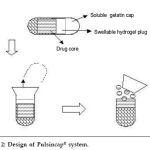 |
Figure 2:
|
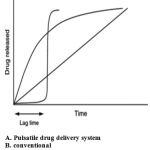 |
Figure 3:
|
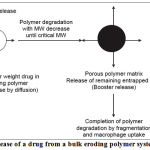 |
Figure 4:
|
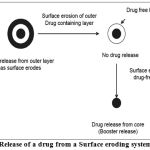 |
Figure 5:
|
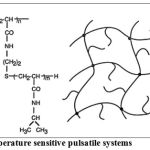 |
Figure 6:
|
Conclusion
Sustained release formulations are not efficient in treating the diseases especially diseases with chronological pathopysiology, for which, pulsatile drug delivery is beneficial. Various methodologies are employed for developing pulsatile drug delivery like time controlled PDDS which includes delivery systems with rupturable coating layer or with erodible coating layers or with release controlling plug, stimuli induced PDDS less temperature induced and chemical stimuli induced systems and externally regulated system. A significant amount of progress has been made towards achieving pulsatile drug delivery systems that can effectively treat diseases with non-constant dosing therapies, such as diabetes. Products that are currently under development for commercialization are for the delivery of proteins, harmones, pain medications and other pharmaceutical medications
References
- W.A. Ritschel, H. Forusz, Chronopharmacology: a review of drugs studies, Meth. Find. Exp. Clin. Pharmacol. 16 (1)(1994) 57– 75.
- T. Bussemer, I. Otto, R. Bodmeier, Pulsatile drug-delivery systems, Crit. Rev. Ther. Drug Carr. Syst. 18 (5) (2001)433– 458.
- B. Lemmer, Ciradian rhythms and drug delivery, J. Control.Release 16 (1991) 63– 74.
- B. Lemmer, Chronopharmacokinetics: implications for drug treatment, J. Pharm. Pharmacol. 51 (1999) 887– 890.
- Gazzaniga, M.E. Sangalli, F. Giordano, Oral chronotopic drug delivery systems: achievement of time and/or site specifity,Eur. J. Pharm. Biopharm. 40 (4) (1994) 246– 250.
- Gazzaniga, P. Iamartino, G. Maffione, M.E. Sangalli, Oral delayed-release system for colonic specific delivery, Int. J.Pharm. 108 (1994) 77– 83.
- I.R. Wilding, S.S. Davis, F. Pozzi, P. Furlani, A. Gazzaniga,Enteric coated timed release systems for colonic targeting, Int.J. Pharm. 111 (1994) 99–102.
- M. Otsuka, Y. Matsuda, Controlled drug release of highly water-soluble pentoxifylline from time-limit disintegrationtype wax matrix tablets, Pharm. Res. 11 (3) (1994) 351–354.
- U. Conte, P. Giunchedi, L. Maggi, M.E. Sangalli, A. Gazzaniga,P. Colombo, A. LaManna, Ibuprofen delayed release dosage forms: a proposal for the preparation of an in vitro/in vivo pulsatile system, Eur. J. Pharm. Biopharm. 38 (6) (1992)209–212.
- L. Maggi, U. Conte, R. Burni, Delivery device for the release of the active ingredient in subsequent times, in: Proceed. Int.Control. Rel. Bioact. Mater., Boston, USA, vol. 26, 1999.
- Y. Ueda, T. Hata, H. Yamaguchi, S. Ueda, M. Kotani, Time controlled explosion system and process for preparation the same, U.S. Patent 4, 871, 549, October 03, 1989.
- B. Amsden, Y.-L. Cheng, A generic protein delivery system based on osmotically rupturable monoliths, J. Control. Release 33 (1995) 99– 105.
- I.E. Lerner, M. Flashner, A. Penhasi, Delayed total release gastrointestinal drug delivery system, WO Patent 99/18938, April 22, 1999.
- Kro¨gel, R. Bodmeier, Floating or pulsatile drug delivery systems based on coated effervescent cores, Int. J. Pharm.187 (1999) 175– 184.
- R. Morita, R. Honda, Y. Takahashi, Development of oral controlledrelease preparations, a PVA swelling controlled release system (SCRS): I. Design of SCRS and its release controllingfactor, J. Control. Release 63 (2000) 297– 304.
- M.E. Sangalli, A. Maroni, L. Zema, C. Busetti, F. Giordano,Gazzaniga, In vitro and in vivo evaluation of an oral systemfor time and/or site-specific drug delivery, J. Control. Release 73 (2001) 103– 110.
- Bussemer, T., Bodmeier, R., 2001. Review of pulsatile drug delivery. Am.Pharm. Rev. 4, 24.
- Bussemer, T., Bodmeier, R., 2003.
- Formulation parameters affecting the performanceT. Bussemer, I. Otto, R. Bodmeier, Pulsatile drug-delivery systems,Crit. Rev. Ther. Drug Carrier Syst. 18 (5) (2001) 433–458.
- B. Lemmer, Circadian rhythms and drug delivery, J. Control Release16 (1991) 63–74.
- T. Bussemer, I. Otto, R. Bodmeier, Pulsatile drug-delivery systems,Crit. Rev. Ther. Drug Carrier Syst. 18 (5) (2001) 433–458.
- B. Lemmer, Chronopharmacokinetics: implications for drug treatment,J. Pharm. Pharmacol. 51 (1999) 887–890.
- W.A. Ritschel, H. Forusz, Chronopharmacology: a review of drugs studies, Methods Find. Exp. Clin. Pharmacol. 16 (1) (1994) 57–75.
- N.A.Peppas,Fundamentals on pH and temperature sensitive deliverysystems Pulsatile Drug Delivery,WissenschaftlicheVerlagsgesellschaft, Stuttgart, 1993, pp. 41–56.
- Sangalli, M.E., Maroni, A., Zema, L., Busetti, C., 2001. In vitro and in vivo evaluation of an oral system for time and/or site-specific drug delivery. J. Control. Release 73, 103–110.
- H.N.E. Stevens, A. Rashid, M. Bakhshaee, Dispensing device, USPatent, 5,474,784 (1995).
- B. Lemmer, Circadian-rhythms and drug delivery, J. Control. Release16 (1–2) (1991) 63–74.
- P. Fantidis, T.P. De Prada, A. Fernandez-Ortiz, A. Carcia-Touchard, F. Alfonso, M. Sabate, R. Hernandez, J. Escaned, C. Bauelos, C.Macaya, Morning cortisol production in coronary heart disease patients, Eur. J. Clin. Invest. 32 (5) (2002) 304–308.
- S.K. Gupta, G. Sathyan, Pharmacokinetics of an oral once- daycontrolled-release oxybutynin formulation compared withimmediaterelease oxybutynin, J. Clin. Pharmacol. 39 (3) (1999) 289–296.
- Davis SS and Illum L (1998) Int. J. pharm. 176:1-8
- Youan B (2004) J. Control. Rel. 98:337-353
- Maroni A, Zema L, Cerea M, et al. (2005) Expt.Opinion Drug Del. 2:855-871
- Pozzi, Furlani, Gazzaniga, et al. (1994) Journal of Controlled Release 31:99-108
- Siegel RA and Pitt CG (1995) J. Control. Rel.33:173-188
- Hermida RC, Ayala DE, Calvo C, et al. (2007) Adv.Drug Del. Rev. (In press)Lemmer (1991) J. Control. Rel. 16:63-74
- Krogel I and Bodmeier R (1999) Int. J. Pharm.187:175-184
- Sungthongjeen S, Puttipipatkhachorn S, Paeratakul O, et al. (2004) J. Control. Rel. 95:147-159
- Thombre AG, Appel LE, Chidlaw MB, et al. (2004)J. Control. Rel. 94:75-89
- Gazzaniga A, Paluga L, Foppoli A, et al. (2007) Eur. J. Pharm. and Biopharm. (In Press)
- Sangalli ME, Maroni A, Foppoli A, et al. (2004)European Journal of Pharmaceutical Sciences469-476
- Jimoh AG, Wise DL, Gresser JD, et al. (1995) J.Control.Rel. 34:87-95
- Kost J and Langer R (2001) Adv. Drug Del. Rev.46:125-148
- Kikuchi A and Okano T (2002) Adv. Drug Del. Rev.54:53-77
- Bae YH, Okano T, Hsu R, et al. (1987) Makromol.Chem. 8:481-485
- Kataoka K, Harada A and Nagasaki Y (2001) Adv.Drug Del. Rev. 47:113-131
- Obaidat AA and Park K (1996) Pharm. Res. 13:989-995
- Obaidat AA and Park K (1997) Biomaterials18:801-806.







