Seyyed Jamaleddin Ebrahim1, Farzam Bidarpoor2, Akbar Eslami3 and Leila Ebrahimzadeh1*
1MSc of Environmental Health Engineering, Kurdistan University of Medical Sciences, Sanandaj, Iran 2Deputy of Health, Kurdistan University of Medical Sciences, Sanandaj, Iran 3Dept. of Environmental health Eng, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Corresponding author email: Leila.e980@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/909
Abstract
The most important types of the subsidiary compounds which are produced as by-products of disinfection process are trihalomethanes, haloacetic acids, haloacetonitriles, chlorites, chlorinated hydrates, formaldehydes, and cyanogen chlorides. Given the adverse effects of disinfection by-product compounds in drinking water, especially the carcinogenic effects of trihalomethanes, the aim of this study was to evaluate the effect of heating the water to reduce or eliminate the amount of trihalomethanes in drinking water in the city of Sanandaj. To conduct this study, 12 samples were collected from the three endpoints of the water distribution network in Sanandaj in September 2015 (the sampling was carried out in places where, according to the results of the previous samplings, had the highest amount of trihalomethanes compared to other parts of the water distribution network). Results were analyzed using Excel statistical software. The results showed that after boilling water for 1 minute, chloroform reduced from 27 to 6.8 µg/l (74.8% removal), after 3 minutes it reduced to 4.1 µg/l (84.8% removal), and finally after 5 minutes it reached zero µg/l (100% removal). After boiling water for 1 minute, dichlorobromomethane reduced from 26 to 7 µg/l (73.1% removal), after 3 minutes it reduced to 4.1 µg/l (87.7% removal), and after 5 minutes it reached zero µg/l (100% removal). After boiling water for 1 minute, dibromochloromethane reduced from 8 to 4.2 µg/l (70% removal), and after 3 minutes it reached zero µg/l (100% removal). After boiling water for 1 minute, bromoform reduced from 8 to 0.9 µg/l (55% removal), and after 3 minutes it reached 0 µg/l (100% removal). Given that chloroform and dichloromethane constitute the highest percentage of trihalomethanes in water samples in Sanandaj, boilling the water, which is a very cost effective method without a need for expensive laboratory equipment and materials, can have a significant impact in the removal of these dangerous chemicals.
Keywords
trihalomethanes; Drinking Water; Sanandaj; Boilling
Download this article as:| Copy the following to cite this article: Ebrahim S. J, Bidarpoor F, Eslami A, Ebrahimzadeh L. Removal of Trihalomethanes from Drinking Water Via Heating Method . Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Ebrahim S. J, Bidarpoor F, Eslami A, Ebrahimzadeh L. Removal of Trihalomethanes from Drinking Water Via Heating Method. Biomed Pharmacol J 2015;9(1). Available from: http://biomedpharmajournal.org/?p=6758 |
Introduction
The most important types of the subsidiary compounds which are produced as by-products of disinfection process are trihalomethanes (THMS), haloacetic acids (HAAS), haloacetonitriles (HANS), chlorites, chlorinated hydrates, formaldehydes, and cyanogen chlorides (1). Trihalomethanes (chloroform, bromodichloromethane, dibromochloromethane and tri bromoform) are the most common by-products of water chlorination which are found in higher concentrations in comparison with other organo halogenated pollutants (2).
These materials are formed as a result of the reaction of chlorine with a number of organic compounds in the water, including natural organic matters in surface waters, such as humic acid and fulvic acid, substances secreted by algae, rural and industrial wastewater, agricultural drainage, and solid waste leachates (3).
The formation of trihalomethanes depends on various factors, including temperature, contact time with chlorine, chlorine concentration, bromine concentration, pH, and the amounts of natural organic matters (4, 5). Rising the temperature leads to increased reaction rate and increased production of trihalomethanes (6). Increasing the retention time (contact with chlorine), and high concentrations of chlorine, bromine, pH, and natural organic matters in the water (as the most important factor) leads to the formation of these compounds (7). Conventional water treatment processes, including clarification, coagulation, flocculation, sedimentation, filtration, and disinfection, can only remove 30% of the precursors of trihalomethanes (8).
Trihalomethanes and haloacetic acids are toxic, carcinogenic, and mutagenic. Carcinogenic effects of trihalomethanes have been demonstrated in the development of the bladder, kidney and intestine cancers (9). According to the World Health Organization’s guidelines for drinking water quality and the Fifth Edition of guidelines by the Institute of Standards and Industrial Research of Iran, respectively, the acceptable level of chloroform is 200 and 300, bromoform is 60, chlorodibromomethane is 100, and bromodichloromethane is 100 µg / l (10, 11).
There are different methods to eliminate or reduce the amount of trihalomethanes including the use of alternative disinfectants and removal of the precursors of trihalomethanes; these methods can prevent the production and dissemination of these compounds in drinking water distribution network and can remove trihalomethanes after their formation (12).
Heating method is one of the methods used to remove trihalomethanes after the formation of these compounds. This method is based on the chemical process of evaporation in which each of the trihalomethanes is removed at a specific boiling point (13).
The conventional methods used for the removal of these compounds, such as the use of activated carbon fibers (14), granular activated carbon (15), membrane processes (16), ultrasonic methods (17), Air Stripping Packed – Column (18), etc. need equipment, chemicals, and certain laboratory conditions (pilot scale, etc.) and hence they are very high cost. Therefore, the utilization of practical, low cost methods, without the use of chemicals, and with high removal efficiency, can improve the applicability of the process.
As the analysis of drinking water samples in Sanandaj proved the presence of trihalomethanes in the water distribution network, this study was aimed to investigate the effects of heating the water on the elimination or reduction of the amount of trihalomethanes in drinking water in the city of Sanandaj.
Materials and Methods
The drinking water for the city of Sanandaj, with a population of 419000 people, is provided from Vahdat dam. The amount of trihalomethanes in the water distribution network of Sanandaj may increase due to the rise in temperature, increased growth of plants and algae in the summer, and also because of pre-chlorination process.
Sanandaj Water Treatment Plant was established and launched in 1983. Sanandaj Water Treatment Plant uses the conventional water treatment processes which includes the following steps and equipments: 1. pre-chlorination, 2. rapid mix basin (ferric chloride is added and using two mixers for each basin the rapid mixing occurs 16 times), 3. settling and sedimentation basin, 4. rapid sand filtration, and 5. gas chlorination.
To conduct this study, 12 samples were collected from the three endpoints of the water distribution network in Sanandaj in September 2015 (the sampling was carried out in places where, according to the results of the previous samplings, had the highest amount of trihalomethanes compared to other parts of the water distribution network).
To collect the samples we used a sterile dark glass container with a capacity of 100 ml. Before sterilizing the container, it was washed several times using detergent and diluted hydrochloric acid. At each sampling site, two samples were taken at the same time. The first sample was poured and kept in the container without any intervention. The second sample was poured in a steel container with a capacity of 2.5 liters and was heated on a home oven. Three samples were taken from the heated water after 1, 3, and 5 minutes of boiling and were poured into sampling container. The samples were stored in the presence of ice and in less than 12 hours were sent to a laboratory with advanced equipment in School of Public Health, Tehran University of Medical Sciences. The samples were analyzed using a gas chromatograph device, CP-3800 model, manufactured by VARIAN Company in Australia. The device was equipped with a flame ionization detector (GC-FID) and a COMBIPAL HS auto sampler. The samples were analyzed based on standard method of 6232 for water and wastewater testing (19).
Results
Based on the results of this study, the concentration of all the four forms of trihalomethanes in the water samples was less than the maximum allowable level set by the Iran National Institute of Standards and World Health Organization. However, they were a little less than the maximum allowable level set by the US Environmental Protection Agency (80 µg/l for all the trihalomethanes) and maximum contaminant level goals (MCLG) set by this agency for each of the trihalomethanes (zero µg/l for dichlorobromomethane and bromoform and 60 µg/l for dibromochloromethane). The most common trihalomethanes, which were observed in drinking water samples taken from the water distribution network in Sanandaj, respectively, were chloroform (43.3%), dichlorobromomethane (41.6%), dibromochloromethane (13.3%) and bromoform (2.8%). Table 1 presents the amount and percentage of each type of trihalomethanes in the three analyzed water samples.
Table 1: Concentration of trihalomethanes in the three drinking water samples collected in Sanandaj (µg/l)
|
Number of the sample |
TCM (%) |
BDCM (%)
|
DBCM (%) | TBM (%) |
THMs |
| 1 | 26 (42.6) | 25 (41) | 8 (13.1) | 2 (3.3) | 61 |
| 2 | 27 (42.9) | 26 (41.3) | 8 (12.7) | 2 (3.2) | 63 |
| 3 | 25 (43.1) | 24 (41.4) | 8 (13.8) | 1 (1.7) | 58 |
| Mean | 26 (43.3) | 25 (41.6) | 8 (13.3) | 1.7 (2.8) | 60.1 |
| SD | 1 | 1 | 0 | 0.58 | 2.5 |
Table 2 and Figure 1 show the changes in the concentration of trihalomethanes in the sample No. 2 (with the highest amount of trihalomethanes) before and after heating. According to the data in presented in Table 2, after boiling water for 1 minute, chloroform reduced from 27 to 6.8 µg/l (74.8% removal), after 3 minutes it reduced to 4.1 µg/l (84.8% removal), and finally after 5 minutes it reached zero µg/l (100% removal). After boiling water for 1 minute, dichlorobromomethane reduced from 26 to 7 µg/l (73.1% removal), after 3 minutes it reduced to 4.1 µg/l (87.7% removal), and after 5 minutes it reached zero µg/l (100% removal). After boiling water for 1 minute, dibromochloromethane reduced from 8 to 4.2 µg/l (70% removal), and after 3 minutes it reached zero µg/l (100% removal). After boiling water for 1 minute, bromo form reduced from 8 to 0.9 µg/l (55% removal), and after 3 minutes it reached zero µg/l (100% removal). Therefore it can be concluded that with increasing the duration of heating the water, the removal rate increases; in addition, with a reduction in the concentration of trihalomethanes in water, their removal through boiling will be performed more quickly.
Table 2: Changes in the concentration of trihalomethanes in the sample No. 2 after heating (µg/l)
| (THMs) | Initial concentration
|
Concentration after boiling / % | ||
| (1min) | (3min) | (5min) | ||
| TCM | 27 | 6.8(74.8%) | 4.1(74.8%) | 0(100%) |
| BDCM | 26 | 7(73.1%) | 3.2(87.7%) | 0(100%) |
| DBCM | 8 | 2.4(70%) | 0(100%) | 0(100%) |
| TBM | 2 | 0.9(55%) | 0(100%) | 0(100%) |
| Total | 63 | 17.1(72.9%) | 7.3(88.4%) | 0(100%) |
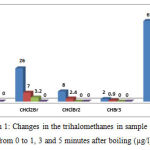 |
Figure 1: Changes in the trihalomethanes in sample No. 2 from 0 to 1, 3 and 5 minutes after boiling (µg/l)
|
Discussion
The results of this research can be explained and justified through a review of the boiling points of trihalomethanes which are equal to 61.2 ˚C for chloroform, 90 ˚C for dichlorobromomethane, 120 ˚C for dibromochloromethane, and 149 ˚C for bromoform (20). Given that chloroform and dichlorobromomethane constitute the highest percentage of trihalomethanes in water samples in Sanandaj, boiling the water can be used as an appropriate method, because it not only has the lowest cost and needs the least laboratory equipment and materials, but also it is effective in removing these dangerous chemicals.
The results of our study are in line with the results of a study by Stewart et al. in which the percentage of the removal of chloroform and dichlorobromomethane after 1, 2, and 5 minutes of boiling the water, respectively, was 75%, 84%, and 97%; however, in our study, after 5 minutes of boiling, both the mentioned forms of trihalomethanes were removed by 100%. The results are also almost similar for dibromochloromethane; in our study, after 1 and 3 minutes of boiling, this material was removed by 70% and 100%, respectively, and in Stewart et al.’s study, after 1, 2, and 5 minutes of boiling it was removed by 70%, 84%, and 100%, respectively. According to the results of Stewart et al.’s study and our study, respectively, after boiling the water for 1 minute, bromoform was removed by 60% and 55%, while after 2, and 3 minutes it was removed by 100% in both studies. These two studies are different in terms of the initial concentration of trihalomethanes, as the initial concentrations of chloroform, dichlorobromomethane, dibromochloromethane, and bromoform, respectively, were 13, 26, 18, and 0.9 µg/l in Stewart et al.’s study and 27, 26, 8, and 2 µg/l in our study (21).
Wells et al. in 2001 investigated the effect of heat treatment on disinfection byproducts in drinking water and concluded that due to the volatility of trihalomethanes, boiling the water can be used an an effective method to change the concentration of these substances in water. According to the results of their research, after 1 minute of boiling the initial concentration of chloroform, which was 569 µg/l, was reduced by 67.3% and after 5 minutes it was reduced by 82%. Nevertheless, the initial concentration of chloroform in Sanandaj water samples was much lower (27 µg/l) and certainly they were expected to show a better removal percentage. The initial concentration of dichlorobromomethane was largely similar between the two studies; its concentration was 25.4 µg/l in Stewart et al.’s study and 26 µg/l and in our research study, and after 1 minute they reduced by 73% and 73.1% and after 5 minutes they reduced by 94.5% and 100%, respectively. The amount of dibromochloromethane was 0.85 µg/l in Stewart et al.’s study and 2 µg/l and in our study was 2 µg/l which were eliminated in the first minute of boiling. In addition, there was no bromoform in the samples analyzed by Stewart et al (22).
Gloria et al. in 2013 investigated the removal of disinfection by-products through heating by means of a kettle, microwave, and pans. In this study, contrary to the results of our study, bromoform constitute the most common form of trihalomethanes in water samples (62%), and chloroform was the least common form of trihalomethanes (4%). Accordingly, after boiling the water in the electric kettle and frying pan, bromoform had the lowest percentage of removal (40% and 64%, respectively) and chloroform had the highest percentage of removal (69% and 78%, respectively). In addition, using microwave, bromoform and chloroform were removed by 97% and 84%, respectively (23).
Given the sharp decline in the water level of the Vahdat dam in Sanandaj, the increase in the temperature, and the growth of algae, the increases of trihalomethanes in drinking water distribution network is inevitable, especially in summer. On the other hand, according to the experts working in Water and Wastewater Company of Sanandaj, it is not possible to remove these materials before chlorination in the water treatment plant. It highlights the need for interventions to minimize the concentration of trihalomethanes in the water distribution network so that to reduce the risk of people’s exposure to these substances through drinking the water. Hence, boiling the water, in every possible way, especially during warm seasons of the year, can be one of the best options.
References
- Etemadi Ali, Tabatabai Ghomsheh said Mostafa., The formation of trihalomethanes and methods for removing them from drinking water. Eighth National Congress of chemical engineering. 2010, Razi University, Kermanshah, Iran.
- Bratby, J., Coagulation and flocculation in water and wastewater treatment. 2006: Intl Water Assn.
- Alicia, C. “DBP formation during chlorination.” J. AWWA, (2000). 92, 76-90.
- Lekkas, T., Environmental Engineering: Management of Water Resources. Univerity of the Aegean, Department of Environmental Studies, Mytilene, Greece, 1996.
- Pourmoghaddas, H. and A.A. Stevens, Relationship between trihalomethanes and haloacetic acids with total organic halogen during chlorination. Water Research, 1995. 29(9): p. 2059-2062.
- Baytak, D., et al., Seasonal variation in drinking water concentrations of disinfection by-products in IZMIR and associated human health risks. Science of the Total Environment, 2008. 407(1): p. 286-296.
- Fazlzadeh Davil M, Mahvi A.H, Mazloomi S3, nabizadeh R, Younesian M, Nazmara S. Concentration of Trihalomethanes in Tehran Drinking Water.
- Asgari, Gh. Ghanizadeh, A. Seyd Mohammadi. Adsorption of Humic Acid from Aqueous Solutions onto Modified Pumice with Hexadecyl Trimethyl Ammonium Bromide. J Babol Univ Med Sci; 14(Suppl 1); winter 2012.
- Koparal A, Yildiz Y.S, Keskinler B, Demircoglu N. Effect of initial pH on removal of humic substances from wastewater by electro coagulation. Sep Purifi Technol_2008; 5 (2):175-82.
- (2006). Guidelines for drinking-water quality: Incorporating first addendum, Vol 1. Recommendations.
- Drinking water –Physical and chemical specifications Institute of Standards and Industrial Research of Iran. revision.2009.
- Nikolaou, Anastasia. 2003. Haloforms and related compounds in drinking water. Springle-Verlag Berlin
- Wells W. Wu, Mark M. Benjamin, Gregory V. Korshin. Effects of Thermal Treatment on Halogenated Disinfection By-Products In Drinking Water.. Wat. Res. Vol.35, No. 15, pp. 3545–3550, 2001.
- Uchid, T. Nakamura, N. Kawasaki, S. Tanada. Adsorption Characteristics of Trihalomethanes ontoActivated Carbon Fiber from Quarternary Mixture Solution. Bull. Environ. Contam. Toxicol. (1997) 59:935-940.
- K. Koumenides, N. Sakkas, D.F. Lekkas, N. Xylourgidis. Using Gac To Control Thms In Drinking Water. An Experimental Study At The Athens Water Works And An Economic Evaluation Of The Method. Global Nest: the Int. J. Vol 3, No 3, pp. 189-197, 2001.
- Vedat Uyak, Ismail Koyuncu , Ibrahim Oktem , Mehmet Cakmakci , Ismail Toroz . Removal of trihalomethanes from drinking water by nanofiltration membranes. Journal of Hazardous Materials 152 (2008) 789–794.
- Khordehdan R., Azimi A., Baghdadi M., Zahedi A., Evaluation of the amount of THMs in drinking water of Bandar Abbas and feasibility study of the removal of THMs via ultrasonic waves. Journal of Water and Wastewater. No. 2. 2014.
- Samadi M. T., Naseri S., Mesdaghinia A., Alizadehfard M. R., Comparative analysis of trihalomethane compounds removal from drinking water using Air Stripping Packed – Column and nanofiltration. Water and Wastewater. No. 57. 2006.
- , AWWA., WEF. Standard methods for the examination of water and wastewater, 20th Ed, (1998).
- Centers for Disease Control and Prevention. International Chemical Safety Cards (ICSC). 2014.
- Stuart W. Krasner, Michael Wright. The effect of boiling water on disinfection by-product exposure. Water Research. Volume 39, Issue 5, March 2005, Pages 855–864.
- Wells W. Wu, Mark M. Benjamin And Gregory V. Korshin. Effects of Thermal Treatment on Halogenated Disinfection By-Products In Drinking Water. Wat. Res. Vol. 35, No. 15, pp. 3545–3550, 2001.
- Glòria Carrasco-Turigas, Cristina M. Villanueva, Fernando Goñi, Panu Rantakokko, and Mark J. Nieuwenhuijsen. The Effect of Different Boiling and Filtering Devices on the Concentration of Disinfection By-Products in Tap Water. Journal of Environmental and Public Health. 2013.








