Lucy Mohapatra1,*, Subrat Kumar Bhattamisra2, 4, Rama Chandra Panigrahy1 And Sambit Kumar Parida3
1 SRF-CSIR; Berhampur University, Berhampur, Odisha, India 2 Dept. of Pharmacology, Roland Institute of Pharm. Sciences, Berhampur, Odisha, India 3 Dept. of Pharmaceutical chemistry, College of Pharm. Sciences, Berhampur, Odisha, India 4Present address: Dept. of Life Sciences, School of Pharmacy, International Medical University, BukitJalil 57000, Kuala Lumpur, Malaysia
DOI : https://dx.doi.org/10.13005/bpj/948
Abstract
Seaweeds have proved to be exceptional resources of nutrients and secondary metabolites. The present study was designed to evaluate the antioxidant, hypoglycaemic and antidiabetic activities of some seaweeds of east coast of India namely Gracilariaverrucosa, Enteromorphacompressa, Ulvafasciata and Turbinariaconoides. Petroleum ether, ethyl acetate and methanol extracts of these seaweeds were evaluated for antioxidant activity by means ofassays like total phenolic content, total reducing power, Nitric Oxideand H2O2scavenging tests.The extracts with superior antioxidant activity among these seaweeds were further tested forhypoglycaemic and antidiabetic activity. Ethyl acetate extract of Ulva fasciata(EAU) has shown promising results after all these tests. IC50 value of EAU was found to be 123.39 and 127.65μg/ml for H2O2and NO free radical scavenging activityrespectively. Total phenol content for EAU was 207.23 ± 2.41mg/g. Fasting plasma glucose level in normal mice was significantly (p<0.05) decreased even after 6 days of EAU treatment at the dose 200mg/kg. The area under curve of oral glucose tolerance test was not affected significantly, though considerable reduction in AUC was found in both EAU 100 and 200 mg/kg treated groups. It was found that EAU has potent in vitro alpha-amylase inhibiting property when compared to the standard, Acarbose. The current findings suggest that,the anti-diabetic activity of U.fasciatamay be due to its underlying antioxidant, hypoglycemic and alpha amylase inhibiting property. Hence, this seaweed can be beneficial for diabetic individuals.
Keywords
Alloxan;Antioxidants; Plasmaglucose;Oral glucose tolerance test; Phenolic;Plasma triglyceride; Plasma total cholesterol
Download this article as:| Copy the following to cite this article: Mohapatra L, Bhattamisra S. K, PanigrahyR. C, Parida S. K. Evaluation of the Antioxidant, Hypoglycaemic and Antidiabetic Activities of Some Seaweed Collected From the East Coast of India. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Mohapatra L, Bhattamisra S. K, PanigrahyR. C, Parida S. K. Evaluation of the Antioxidant, Hypoglycaemic and Antidiabetic Activities of Some Seaweed Collected From the East Coast of India. Biomed Pharmacol J 2016;9(1). Available from: http://biomedpharmajournal.org/?p=6576 |
Introduction
Diabetes mellitus is a cluster of never-ending diseases, which is recognized by its most common characteristic, hyperglycaemia. It is usually classified in to two main categoriesi.e. Type I diabetes mellitus (T1DM), caused due to complete absence of insulin production and Type II diabetes mellitus (T2DM), due to the relative deficiency of insulin secretion and tissue resistant to the insulin action1.Majority of this disease all over the world may be correlated to modern diet, obesity and modern sedentary lifestyle. The mortality associated with diabetes is mainly due to the increased risk of several complications of this disease. Very often the most common life threatening complications includes hypertension, retinopathy, nephropathy and other cardio vascular diseases2. These incidences necessitate and warrant the search for new preventive measures as well as healing strategy of this disease.
Traditionally, several seaweeds have been consumed as food in many parts of the world especially among Asiancoastal communities. According to some report, Japanese consume approximately 5.3 g seaweed in their daily diet3. In addition to this, seaweed has also been reported to be used against numerous disease causing ailments in different Asian traditional medical systems4. Some studies have revealed that, regular consumption of seaweed lowers menace of several diseases like cardiovascular disease, hyperlipidaemia and breast cancer5,6. Furthermore some studies have also established the fact that, reduction of seaweed consumption in some Asian societies that regularly used to consume seaweed due to an enhanced yearning for Westernised diet, augmented occurrence of unending lifestyle diseases6. These studies highlight the promising health benefits offered by seaweed. It’s true that despite of these proven facts, this generous treasure of the ocean has been ignoredsince long.Even today they are the same under-utilised and untapped resources of diversified secondary bio molecules having potential therapeutic applications, normallyabsent in terrestrial plants.
In this study, we aim to communicate the beneficial utility of some seaweed obtained from Indian east coast in preventing and managing diabetes via various pharmacologically relevant attestations.Seaweed flora is represented by three major classes’ namely green, brown and red algae which differ from one another in their morphology, life cycle, distribution, pigment and secondary metabolite composition. It was decided by us to take one or two species from each class to assess their antidiabetic potential. During the course of study one species of red algae Gracilariaverrucosa(Huds.) Papenfuss (Family: Gracilariaceae), two species of green algae Eenteromorphacompressa(L.) Grev. (Family: Ulvaceae) and UlvafasciataDelile (Family: Ulvaceae) were collected from the Chilika Lake, Odisha, Indian east coast. Due to non-availability of brown seaweed along the Odisha coast, one of the species from this variety Turbinariaconoides(J. Agardh) Kutzing (Family: Sargassaceae) was obtained from the Palk Bay and Gulf of Mannar, Tamilnadu, Indian east coast. All seaweeds were properly identified and authenticated by Prof. R.C. Panigrahy, marine taxonomist, Berhampur University, Berhampur, Odisha, India.
From some of the studies it is confirmed that a sufficient use of antioxidant may prevent or delay β-cells dysfunction in diabetes and prevent the development of complications associated with this disease7,8. Hence, initially all of these seaweed extracts were investigated for their probable antioxidant property. Those extracts showing goodresultsin this preliminary investigation, were further evaluated for their in vivo hypoglycaemic and antidiabetic properties in normal and diabetes induced animals respectively.
Materials And Methods
Materials
Only analytical grade chemicals were used for this investigation. Streptozotocin (STZ), Ethylene diamine tetra acetic acid (EDTA) and haematoxylin–eosin (H&E) dye were purchased from Himedia Lab. and Loba Chemie Pvt. Ltd., India. All other solvents and chemicalswere procured from Merck Specialties Pvt. Ltd., India. Biochemical parameters were investigated by using glucose kit (GOD/POD method), triglycerides kit (GPO/PAP method) and cholesterol kit (CHOD/PAP method)which were obtained fromCrest Biosystems, India.
Seaweed extraction
Seaweeds collected from different places were carefullywashed with sea water and subsequently in distilled water to get rid of all debris and impurities. These seaweeds were then air dried and stored in air tight polyethylene bags for further use. Required amount of each of the seaweed was cut into small pieces with the help of sharp knife and powdered with the help of electronic grinder separately. These powders were sievedby using No.16 mesh. Exactly, 1000g of each seaweed powder was extracted separately in soxhlet apparatus in the increasing order of polarity using three solvents i.e petroleum ether (pet. ether), ethyl acetate and methanol9,10. All the extracts obtained were encoded as following:
PEG, EAG & MEG: pet. ether, ethyl acetate & methanol extract of Gracilariarespectively.
PEE, EAE & MEE: pet.ether, ethyl acetate & methanol extract of Enteromorpharespectively.
PEU, EAU & MEU: pet. ether, ethyl acetate & methanol extract of Ulvarespectively.
PET, EAT & MET: pet. ether, ethyl acetate & methanol extract of Turbinaria respectively.
Phytochemical analysis of extracts
The extracts obtained were analyzed for presence of different phyto constituents using established validated qualitative methods9,10.
Table 1: Phytochemical composition of petroleum ether, ethyl acetate and methanol extracts of different seaweeds.
| Constituents | E. compressa | U. faciata | T. conoides | G. verrucosa | ||||||||
| PE | EA | ME | PE | EA | ME | PE | EA | ME | PE | EA | ME | |
| Alkaloids | – | – | – | + | – | – | – | – | – | – | – | – |
| Carbohydrates | – | – | – | – | – | – | – | – | – | – | – | – |
| Gum & mucilage | – | – | + | – | – | + | – | – | – | – | – | – |
| Protein & amino acid | – | – | + | – | – | + | – | – | + | – | – | + |
| Terpenoids | – | – | – | – | + | – | – | + | – | – | + | – |
| Saponins | – | – | – | – | – | – | – | – | + | – | – | – |
| Flavonoids | – | + | + | – | + | + | – | + | + | – | – | + |
| Tannins | – | – | + | – | – | + | – | – | + | – | – | + |
| Glycosides | – | + | + | – | + | + | – | + | + | – | + | – |
| Steroids | + | – | – | + | – | – | + | – | – | – | – | – |
| Phenolic compounds | – | + | + | – | + | + | – | + | + | – | + | + |
PE, petroleum ether extract; EA, ethyl acetate extract; ME, methanol extract; (+): present; (-): absent.
In vitro antioxidant evaluation of extracts
The antioxidant property of each extract was evaluated following the standard methods described below. The chemical tests were performed in triplicate for each seaweed extract.
Determination of total phenol content:
Total phenolic content was determined by Folin-ciocalteu reagent method using Gallic acid as the standard for phenolic compound11. In this method, initially 5 ml of the reagent was mixed with 1 ml of Gallic acid at different concentrations (50, 100, 150, 200 μg/mL) and to which 4 ml of 2% Sodium carbonate was added after 3 minutes. Thirty minutes later, the absorbance of the blue colour was measured at 760 nm. The concentrations of total phenols were expressed as mg/g of dry extract12.
Total reducing power:
The seaweed extracts (50, 100, 150 and 200 μg/ml) prepared in distilled water and 1% potassium ferric cyanide were mixed with phosphate buffer (0.2 M, pH 6.6) and incubated at 50°C for 20 min. Then, 2.5 ml of 10% TCA was added to this reaction mixture and centrifuged at 1000 × g for 10 min. The upper layer of the solution (2.5 ml) was mixed with 2.5 ml of distilled water and FeCl3 (0.5 mL, 0.1%). The absorbance was measured at 700 nm. BHT (50 to 200μg/ml) was used as positive control. The higher the absorbance of the reaction mixture, the greater is its reducing power. Total reducing capacity of seaweed extracts was determined adopting a most widely used method13.
Nitric oxide radical scavenging activity:
In this test, 3 ml of the reaction mixture containing10 mM sodium nitroprusside and different seaweed extracts (50, 100, 150, 200 μg/ml) in phosphate buffer were incubated at 25°Cfor 150 min. Then, 0.5 ml of each incubated reaction mixture was mixed with 1 ml of sulfanilic acid reagent (0.33%in 20% glacial acetic acid) and allowed to stand for 5 minfor complete diazotization. Then 1 ml of naphthylethylenediaminedihydrochloride (0.1%) was added and thesolution was mixed thoroughly. The mixture was allowed to stand for 30 min at 25°C.A pink colored chromophore is formed in diffused light.The absorbance of pink colour solution was measured at 540nm against the corresponding blank solutions. BHT (50-200μg/ml) was used as positive control. The nitric oxide scavengingactivity of the seaweed extracts is reported as percentage inhibition and was calculated as per established method14.
Nitric oxide scavenging effect = (Acont – Atest)/Acont × 100 (Eq. 1)
Where Acontwas the absorbance of the control reaction and Atest was the absorbance in the presence of the seaweeds extracts.
Hydrogen peroxide (H2O2) radical scavenging activity:
H2O2 scavenging activity of different seaweeds extracts was determined adopting standard procedures15. Initially, 40mM of H2O2 was prepared in phosphate buffer (pH 7.4) and the concentration of H2O2 in it was determined spectrophotometrically at 230 nm. The seaweed extracts of 50, 100, 150, 200μg/ml concentrations in distilled water were prepared. Ascorbic acid of same concentrations was also prepared separately. Then 0.6 ml of 40 mM H2O2 solution was added after the lapse of 10 min. Absorbance of the mixture was determined at 230 nm against a blank solution containing phosphate buffer without H2O2. The percentage scavenging of H2O2 was calculated as per Eq. 1.
In vivo hypoglycaemic and antidiabetic study
Experimental animals:
Animal experiments were designed and conducted in accordance with the policies of committee for the purpose of control and supervision of experiments in animal (CPCSEA), India. The experimental protocol was approved (no. 66, dated 7/06/2012) by institutional animal ethics committee (IAEC), Roland Institute of Pharmaceutical Sciences, Berhampur, Odisha, India. Swiss albino mice (22-27 g) were housed in standard cages (48×35×22 cm) at room temperature (20 ± 2°C), relative humidity (55-60%) and a 12 h light/dark cycle. Theywere fed with normal chow pellet, and water ad libitum.
Toxicity study:
The extracts i.e. EAU, EAT and EAG were selected based on their potential antioxidant activity. The extracts of the seaweed Enteromorpha compressa were excluded from further in vivo evaluation due to their poor antioxidant property. Toxicity study of these selected extracts on female Swiss albino mice was carried out as per OECD regulations 42316. The highest dose (i.e. 2000 mg/kg) of each extract was administered orally to the overnight fasted mice. The signs of possible toxicity were observed at every 3 hours for the first 24 hours and every day for 14 days. Individual animal weight was noted down daily and any signs or symptoms of toxicity and mortality was observed for 14 days as described in previous studies17.
Hypoglycemicactivity in normal mice:
Animals were grouped as follow and administered the respective treatment intraperitoneally (i.p.). Group 1: 1% sodium CMC; Group 2: EAU (200 mg/kg); Group 3: EAT (200 mg/kg) and Group 4: EAG (200 mg/kg). Each group consists of fiveanimals. Animals were fasted overnight for a period of 12 h. After the single i.p. injection of the respective extracts, blood sample (0.1 ml) was collected in micro tubes previously filledwith 10% EDTA solution (20 μl of 10% EDTA/ ml of blood). Blood was collected through retro orbital route under mild ether anesthesia at 0, 0.5, 1, 2, 4, 6, 10 and 24 h. The micro tubes were centrifuged at 4000 rpm at 4°C for 20 min to obtain clear plasma. The plasma was thenanalyzed for glucose in the auto analyzer (3000 Evolution, BSI Italy) using commercially available glucose estimation kits. The data is expressed as the percentage change of blood glucose obtained at different time points18.
Antidiabetic activity:
Antidiabetic study was carried out for EAU, which showed the best hypoglycaemic activity as compared to the other extracts.
Diabetes induction and grouping of animals:
Alloxan monohydrate (150 mg/kg, i.p.) was injected to the overnight fasted male Swissalbino mice19. Hyperglycaemia was confirmed by the elevated plasma glucose levels (>180 mg/dL) after 3 days of the induction. The diabetic mice were randomized into 5 different groups and the treatment was administered orally for six consecutive days as follows. However, one group was kept as non-diabetic control without drug treatment (Group 1); Group 2: diabetic control, 1% sodium CMC; Group 3: EAU (100 mg/kg); Group 4: EAU (200mg/kg); and Group 5: Gliclazide (GLI) (10 mg/kg). Each group consists of five animals. At the end of sixth day, blood sample was collected from the retro orbital route of each mouse and biochemical parameters such as plasma glucose, triglyceride and total cholesterol was estimated.
Oral glucose tolerance test (OGTT):
OGTT was performed in overnight (18 h) fasted mice with water ad libitum20,21. On 6th day of treatment, glucose (2 g/kg) was fed to each animal 10 min after collecting blood sample which was taken as reference (0 min). Exactly 0.1 mL of blood was withdrawn from the retro orbital route of each mouse under mild ether anaesthesia at 30, 60 and 120 min after the glucose load. The blood samples were centrifuged to obtain clear plasma which was then analyzed for glucose by using commercially available biochemical kits.
In vitro α-amylase enzyme inhibition assay
The α-amylase inhibition activity of EAU was determined by following a established method22. Starch azure (2mg) was suspended in each of tubes containing 0.2ml of 0.5M Tris-HCL buffer (pH 6.9) and 0.01M CaCl2. The tubes containing substrate solution were boiled for 5 min and were then incubated at 37ᵒC for 5 min. Seaweed extract (0.2ml) was taken in each tube containing different concentrations (20, 40, 60, 80 and 100 µg/ml). Porcine Pancreatic amylase (PPA) was dissolved in Tris-HCL buffer to form a concentration of 2units/ml and 0.1ml of this enzyme solution were added to each of the above mentioned tubes. The reaction was carried out at 37ᵒC for 10 min and was stopped by adding 0.5ml of 50% acetic acid in each tube. The reaction mixture was centrifuged at 3000 rpm for 5 min at 4ᵒC. The absorbance of the resulting supernatant was measured at 595 nm. The α-amylase inhibition activity was calculated as follows:
Alpha-amylase inhibition activity = [(Ac+)-(Ac-)]-[(As-Ab)/ [(Ac+)-(Ac-)]×100 ——–Eq. 2
Where Ac+, Ac-, As are the absorbance of 100% enzyme activity (only solvent with enzyme), 0% enzyme activity (only solvent without enzyme activity), a test sample (with enzyme) and a blank (a test sample without enzyme), respectively.
Statistical analysis
Results were expressed as mean ± SEM. Data were analyzed with One-way Analysis of Variance (ANOVA), followed by Tukey’s multiple comparison tests. The level of significance was set at p< 0.05.
Results
Preliminary phytochemical analysis of different extracts
The detail results of qualitative preliminary phytochemical assays of different seaweed extracts are illustrated in Table 1. Presence of alkaloid was detected in PEU. Gum and mucilage was detected in two green seaweed extracts (MEU and MEE). Tannins, protein and amino acids were detected in methanol extracts of all seaweeds. Phenolic compounds were detected in ethyl acetate and methanol extracts of all seaweeds. Whereas, carbohydrate was absent in all the seaweed extracts.
In vitro antioxidant evaluation of extracts
Table 2: Total phenolic content of ethyl acetate and methanol extracts obtained from seaweeds.
| Seaweed extracts |
Total phenolic content/ gm (mg gallic acid equivalent/ gm of extract) |
| EAU | 207.23 ± 2.41 |
| EAT | 55.71 ± 3.65 |
| EAG | 35.86 ± 3.77 |
| EAE | 31.77 ± 4.23 |
| MEU | 38.82 ± 2.21 |
| MET | 36.77 ± 3.17 |
| MEG | 29.80 ± 3.77 |
| MEE | 25.03 ± 4.91 |
Each experiment was performed in triplicte and the results are expressed as mean ± S.E.M.
EAE: ethyl acetate extract of Enteromorpha; MEE: methanol extract of Enteromorpha; EAU: ethyl acetate extract of Ulva; MEU: methanol extract of Ulva; EAT: ethyl acetate extract of Turbinaria; MET: methanol extract of Turbinaria; EAG: ethyl acetate extract of Gracilaria; MEG: methanol extract of Gracilaria.
Total phenol content:
The total phenol content expressed as gallic acid equivalents was found to be highest for EAU (207.23 ±2.41mg/g) compared to the other extracts as represented in Table 2. The lowest phenol content was found in MEE (25.03 ± 4.91 mg/g). In general, phenolic compounds were found in all the three groups’ i.e. brown, green and red seaweeds taken in the present study.
 |
Figure 1: Total reducing activity of seaweed extracts (50- 200 µg/mL) and BHT (50–200 µg/mL). |
Each experiment was performed in triplicte and the results are expressed as mean ± S.E.M.
EAE: ethyl acetate extract of Enteromorpha; MEE: methanol extract of Enteromorpha; EAU: ethyl acetate extract of Ulva; MEU: methanol extract of Ulva; EAT: ethyl acetate extract of Turbinaria; MET: methanol extract of Turbinaria; EAG: ethyl acetate extract of Gracilaria; MEG: methanol extract of Gracilaria.
Total reducing power:
Reducingcapacity of seaweed extracts and BHT (50-200 µg/mL) is given in Figure 1. EAU showed higher reducing ability at all concentrations compared to other seaweed extracts. At 200 µg/mL the reducing activity of EAU was found to be (absorbance, 1.18 ± 0.006), which was almost nearer to the value recorded for standard BHT (absorbance, 1.48). The reducing ability of methanol extracts of E.compressa and G. Verrucosawas found to be the lowest as compared to the other seaweed extracts.
IC50 value of seaweeds extracts in H2O2 and nitric oxide scavenging activity:
The IC50 value of hydrogen peroxide and nitric oxide free radical scavenging activity is presented in Table 3. The lowest IC50 values for both H2O2 and nitric oxide scavenging activitywas encountered with EAU suggesting thereby that it has potent antioxidant activity compared to other seaweed extracts. The rank order of IC50 value of H2O2 and Nitric oxide scavenging activity of seaweeds are EAU> EAT> MEU> MET> EAG> EAE> MEG> MEE.
Table 3: IC50 value of hydrogen peroxide and nitric oxide free radical scavenging activity of ethyl acetate and methanol extracts obtained from seaweeds.
| Seaweed extracts | IC50 of H2O2 scavenging activity (µg/mL) | IC50 of NO radical scavenging activity (µg/mL) |
| EAU | 123.39 | 127.65 |
| EAT | 141.35 | 164.99 |
| EAG | 168.96 | 258.06 |
| EAE | 181.12 | 283.69 |
| MET | 163.31 | 249.65 |
| MEU | 157.79 | 240.94 |
| MEG | 195.30 | 306.39 |
| MEE | 211.01 | 313.97 |
| Ascorbic Acid | 109.30 | 97.33 |
Each experiment was performed in triplicte and the results are expressed as mean ± S.E.M.
EAE: ethyl acetate extract of Enteromorpha; MEE: methanol extract of Enteromorpha; EAU: ethyl acetate extract of Ulva; MEU: methanol extract of Ulva; EAT: ethyl acetate extract of Turbinaria; MET: methanol extract of Turbinaria; EAG: ethyl acetate extract of Gracilaria; MEG: methanol extract of Gracilaria.
Toxicity study of the seaweed extracts as per OECD guidelines 423:
We observed no significant toxic signs or death during the 14 day observation period. None of the mice showed clinical toxic signs such as anorexia, depression, lethargy and also no mortality happened throughout the examination.
Table 4: Effect of EAU on fasting plasma glucose, triglyceride and total cholesterol in alloxan induced diabetic mice.
| Treatments | Glucose (mg/dl) | Triglyceride (mg/dl) | Total Cholesterol (mg/dl) | |||
| Before | After | Before | after | Before | After | |
| Group 1(N) | 92.0 ± 4.4 | 91.4 ± 3.9 | 35.8 ± 8.3 | 36.0 ± 5.1 | 34.2 ± 6.0 | 36.8 ± 7.6 |
| Group 2(D) | 211.4 ± 20.4# | 226.6 ± 19.8# | 57.0 ± 12.9 | 56.2 ± 14.8 | 58.0 ± 11.0 | 78.0 ± 8.5# |
| Group 3 (EAU1) | 218.2 ± 18.3 | 211.4 ± 16.3 | 46.2 ± 11.8 | 40.4 ± 3.8 | 69.8 ± 6.8 | 57.2 ± 7.3 |
| Group 4 (EAU 2) | 235.2 ± 14.7 | 214.0 ± 10.8£ | 51.4 ± 9.2 | 36.2 ± 4.8 | 64.0 ± 4.2 | 44.4 ± 5.9£ |
| Group 5 (G) | 222.6 ± 18.7 | 176.0 ± 18.4€ | 31.8 ± 6.0 | 29.6 ± 2.8 | 61.6 ± 16.8 | 56.2 ± 10.3 |
N: Normal control, D: Diabetic control, 200 mg/kg, EAU1: Ethyl acetate extract of Ulva sp. 100 mg/kg, EAU2: Ethyl acetate extract of Ulva sp. 200 mg/kg, G: Gliclazide 10 mg/kg. #: p<0.05w.r.t Gr.1; €: p<0.01w.r.t Gr.2; £: p<0.05w.r.t Gr.2. n= 5 in each group.
In vivo hypoglycaemic and antidiabetic study
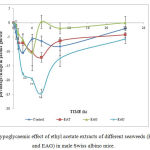 |
Figure 2: Hypoglycaemic effect of ethyl acetate extracts of different seaweeds (EAU, EAT and EAG) in male Swiss albino mice. |
*p< 0.05 and **p< 0.01 vs control; EAU: ethyl acetate extract of Ulva; EAT: ethyl acetate extract of Turbinaria; EAG: ethyl acetate extract of Gracilaria.
Hypoglycaemic activity in normal mice:
Hypoglycaemic effect of EAU, EAT and EAG ate 200 mg/kg is presented in Figure 2. It has been observed that EAU showed the highest hypoglycaemic effect in comparison to other seaweed extracts. Hypoglycaemic effect of seaweeds extracts follows as EAU>EAT>EAG. Maximum effect was observed in between 4 – 6 h of extract administration. EAU treated mice showed -20% and -25% change in plasma glucose at 4 and 6 h respectively. The least change (-10%) in plasma glucose was observed in case of EAG treated mice at 4 h.
Plasma glucose, triglyceride and total cholesterol:
Fasting plasma glucose, triglyceride and total cholesterol was estimated before and after the drug treatment. EAU (200 mg/kg) treated animals showed significant (p < 0.05) reduction in their plasma glucose level after the treatment. However, GLI (10 mg/kg) treated mice showed the maximum reduction (p < 0.01) in plasma glucose as compared to other treatment groups. Fasting plasma triglyceride level in all control and treated animals did not showed significant changes before and after treatment. Plasma total cholesterol was measured before and after the drug treatment for all the groups. EAU at 200 mg/kg showed significant (p < 0.05) reduction in total cholesterol level after treatment. The results are summarized in Table 4.
Oral glucose tolerance test (OGTT):
The extent of reduction in AUC of OGTT was higher in Gliclazide (50 mg/kg) treated mice (p<0.01). The EAU (100 and 200 mg/kg) treated animals though showed decreased trend in the AUC as compared to the diabetic groups but, it was not found to be significant. The AUC of the plasma glucose disappearance curve is shown in Figure 3.
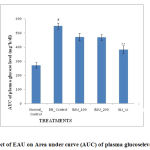 |
Figure 3: Effect of EAU on Area under curve (AUC) of plasma glucoselevelin OGTT. |
DB control, diabetic control; EAU: ethyl acetate extract of Ulva; GLI: Gliclazide; #p< 0.05 and ##p < 0.01 vs. normal control; *p < 0.05 and **p < 0.01 vs. diabetic control.
In vitro α-amylase inhibitory assay
In the present study, the results of in vitro α-amylase inhibitory assay showed that Ulva sp.exhibited a potent inhibitory activity on α-amylase enzyme (IC 50 = 69.122µg/ml)compared with the positive standard, acarbose (IC 50 = 49.344 µg/ml) (Table 5). A concentration dependent inhibition was observed for various concentrations of these algal extracts. The percentage inhibitory activity for EAU was found to be 60.191 ± 2.235 (at 100µg/mL). The percentage inhibitory activity for acarbose at the same concentration was observed as 75.733 ± 0.068.
Table 5: IC50 (50 % inhibition) value of alpha amylase inhibiting activity of EAU.
| Seaweed extracts | IC50 of alpha amylase inhibiting activity (µg mL–1) |
| EAU | 69.122 |
| Acarbose | 49.344 |
EAU: ethyl acetate extract of Ulva
Discussion
Marine macro algae are recognized as potential source of several important secondary metabolites including alkaloids, phenols, flavonoids, saponins, steroids and other related active metabolites.These secondary metabolites participate extensively in their defence against diseases, microorganism, stress and interspecies protections.They have been extensively studied for their importance in furnishing good health and defend against diseases and thus seaweeds could definitely behave as budding hypoglycemic and antidiabetic agents23.Therefore, priliminary phytochemical screening serves as the opening step in predicting the types of probable active compounds present in these seaweed extracts so that the best ever extracts among them could be selected for further studies.The results of the preliminary photochemical study revealed that ethyl acetate and methanol extracts of almost all the seaweeds showed the presence of some of the most important secondary metabolites like terpenoid, flavonoids and phenolics. These metabolites have been proven to provide encouraging antioxidant and anti-diabetic properties24,25.
Some studies reveal the fact that ROS leads to cell and tissue dysfunction and injury caused by glucolipotoxicity in diabetes26,27. In addition to that studies have also proven that, antioxidants can bring to bearvaluable effects on pancreatic β-cells function in diabetes by providing security against glucose toxicity8. Thus, anadequateuse of antioxidants may be valuable in counteracting diabetic complications and managing the disease. In this study, the extracts containing suitable metabolites (Terpenoid, flavonoids, amino acids and phenolics) were evaluated for potential antioxidant activity using established in vitro assays. The results of the present study revealed that ethyl acetate extracts of all the seaweeds exhibited considerable free radical scavenging activity. The reason behind this may be due to the presence of considerable amount of phenolics in them as some studies have claimedthat there exist a positive correlation between total phenolic content and antioxidant property28,29. Based on this fact, EAU, EAT and EAG were studied for hypoglycaemic effect in normal Swiss albino mice as they showed good total phenolic content and prior study has reported that phenolic components present in seaweeds exert potential hypoglycaemic effect30. The finding of this study leads to infer that EAU has potent hypoglycemic effect in normal mice and hence this extract was selected for further antidiabetic study as compounds with hypoglycemic principle have got more direct role in antihyperglycemic activity31.
The chiefobjective in the management of diabetes mellitus is to preserve normal plasma glucose levels in both the fasting and postprandial state. In the present study, EAU has effectively lowered and normalised the blood glucose level at the higher dose. Notably, this effect was significantly prominent and glucose level was pragmatic to be low even on the sixth day of the treatment. However, promising effects were not reflected by AUC of OGTT curve. OGTT reflects the competence of the body to dispose of glucose after an oral glucose load or meal. This test closely mimics the glucose and insulin dynamics of physiological conditions more closely. Impaired glucose tolerance is reflected in a larger incremental AUC of the plasma glucose disappearance curve. Results of OGTT revealed that AUC significantly (p<0.01) increased in diabetic control compared to non diabetic control. Whereas, AUC was lowered in the other treatment groups as compared to diabetic control. EAU (100 and 200 mg/kg) treated animals though showed a decrease in the AUC as compared to the diabetic groups, it was not significant. The important salutary approach to reduce postprandial glucose elevation is to repress the production and/or assimilation of glucose from the gastrointestinal tract. The latter effect can be achieved through inhibition of some enzymes like α-amylase or α-glucosidase, those play important role in glucose absorption form the g.i.t.32.In our investigation we found that ethyl acetate extract Ulva sp. moderately inhibited α-amylase. Hence, the prevailing antidiabetic and hypoglycaemic effect of this extract may be due α-amylase inhibiting property of this extract. In order to establish the real mechanism behind these activities and to find out the actual active compounds responsible in imparting such activities, an extensive study is required.
Conclusion
In conclusion the ethyl acetate extract of the marine macro algaU. Fasciatashowed considerable antidiabetic activity compared to other seaweed extracts. The underlying reason behind this marked activity may be related to its significant antioxidant, hypoglycaemic and in vitro alpha amylase inhibiting activities. However, more comprehensive in vitro and in vivo studies are needed to elucidate the exactmechanism of its antidiabetic activity. Further, the active principle responsible for such activities is required to be isolated andstudied. Acknowledgements
The corresponding author expresses deepest sense of gratitude to the Centre of Scientific and Industrial Research (CSIR), Govt. of India, for providing financial support in the form of senior research fellowship to carry out this work. We express our sincere thanks to the Principal, Roland Institute of Pharmaceutical Sciences, Berhampur, Odisha, India, for providing necessary facilities to carry out the animal studies.
Authors’ Contribution
LM, SKB and RCP have conceived and designed this experimental protocol. LM and SP performed the experiment. SKB and RCP analysed the final data. LM and SP wrote the manuscript.
Conflict of interest disclosure: The authors declare that there is no conflict of interest.
References
- Cefalu, W.T. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: Rationale and specific agents. Pharmacol. Ther., 2007;81: 636–49.
- American Diabetes Association Standards for medical care in diabetes.Diabetes Care, 2008; 31:S12–S54.
- Matsumura, Y. Nutrition trends in Japan.Asia Pac. J. Clin. Nutr., 2001;10:S40–S47.
- Sayad, A.N., Shunmugiah, K.P., Kasi, P.D. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm. Biol., 2013; 51: 1401-10.
- Kim, J., Shin, A., Lee, J.S., Youn, S., Yoo, K.Y. Dietary factors and breast cancer in Korea: An ecological study.Breast J., 2009;15: 683–6.
- Iso, H. Lifestyle and cardiovascular disease in Japan. Thromb., 2011;18: 83-8.
- Wu, X.J.,Hansen,C. Antioxidant capacity, Phenolic content,Polysaccharide content of Lentinusedodesgrown in Wheypermeate-based submerged culture. Food. Sci. 2008; 73: M1-M8.
- Kaneto, H., Kajimoto, Y., Miyagawa, J., Matsuoka, T., Fujitani, Y., Umayahara, Y., Hanafusa, T., Matsuzawa, Y., Yamasaki, Y., Hori,M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999; 48: 2398-406.
- Kokate, C.K., Purohit, A.P., Gokhale, S.B. (ed): A Text Book of Pharmacognosy, 39th edn. Pune, India: Nirali Prakashan, 2007; pp 108-
- Mukherjee, P.K.: Phytoconstituents and their analysis. In: Quality Control of Herbal Drugs-An approach to evaluation of botanicals. 1st ed. New Delhi: Business Horizons, 2002; pp 256-70.
- Singleton, V.L., Orthofer, R.Lamuela-Reventos, R.M.: Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Methods in Enzymology, oxidants and antioxidants – Part A(Packer L. ed). Vol. 299, San Diego, CA: Academic press; 1999; pp152–
- Kim, D.O., Jeong, S.W., Lee,C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Chem., 2003; 81, 321-6.
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. J. Nut.,1986;44: 307-15.
- Rai, S., Wahile, A., Mukherjee, K., Saha, B.P.Mukherjee, P.K.Antioxidant activity of Nelumbo nucifera(sacred lotus) seeds. Ethnopharmacol.,2006;104, 322-7.
- Gulcin, I., Oktay, M., Kirecci, E.Kufrevioglu,O.Screening of antioxidant and antimicrobial activities of anise (Pimpinellaanisum) seed extracts.Food Chem., 2003; 83: 371-82.
- OECD guidelines forthetesting of chemicals (Acute oral toxicity-up & down procedure). The Organization of Economic Co-operation Development, Paris, Adopted 23rd March 2006. [cited, 2008 June 20]; Available from, URL,www.oecd.org.
- Lee, J.N., Park, C.S., Kim, H.P., Hwang, S.Y.Chung,W.G. Single dose toxicity study of Hwangjaegongjinbo, an invigorator, in mice and rats. Toxicol. Pub. Health.,2002; 18: 73-7.
- Syiem D., Monsang Sh.W., Sharma, R. Hypoglycemic and anti-hyperglycemic activity of curcuma amada in normal and alloxan-induced diabetic mice. Pharmacologyonline, 2010; 3: 364-72.
- Okokon, J.E., Bassey, A.L., Obot, J. Antidiabetic activity of ethanolic leaf extract of Croton zambesicus (Thunder plant) in alloxan diabetic rats.Afr.J.Tradit. Complement. Altern. Med., 2006;3: 21-6.
- Barik, R., Jain S., Quatra, D., Joshi, A., Tripathy, G.S., Goyal, R. Antidiabetic activity of aqueous root extract of Ichnocarpusfruitescens in streptozotocin-nicotinamide induced type-II diabetes in rats. J. Pharmacol.,2008;40: 19-22.
- Bhattamisra, S.K., Mohapatra, L., Panda, B.P., Parida, S.Effect of isoflavone rich extract of soya seed extract on glucose utilization and endurance capacity in diabetic rat.croat.,2013;42, 42-52.
- Hansawasdi, C.,Kawabata, J., Kasai,T. Alpha-amylaseinhibitors from Roselle (Hibiscus sabdariffa)Tea.Biosci.Biotechnol.biochem.,2000;64: 1041-3.
- Krishnamurthy, V.: Edible seaweeds. In: Souvenir,Natl Symposium mar. Plants. Their Chemistry and Utilization. 2005;pp 1-4.
- Tanaka, T.Cancer chemoprevention by natural products. Reports,1994;1, 1139-55.
- Lu, X., Chen,, Dong, P., Fu, L., Zhang, X. Phytochemical characteristics and hypoglycaemic activity of fraction from mushroom Inonotusobliquus.J. Sci. Food Agric. 2010;90: 276-80.
- Wolff, S.P., Jiang, Z.Y., Hunt, J.V. Protein glycation and oxidative stress in diabetes mellitus and ageing.Free Radic. Biol. Med.,1991;10: 339-52.
- Baynes, J.W., Thorpe, S.R.Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes,1999;48, 1-9.
- Kuda, T., Tsunekawa, M., Hishi, T., Araki, Y.Antioxidant properties of dried ‘kayamo-nori’, a brown algaScytosiphonlomentaria(Scytosiphonales, Phaeophyceae). Chem.,2005;89,617-22.
- Ganesan, P., Kumar, C.S.,Bhaskar, N.Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Technol.,2008;99:2717-23.
- Lamarche, B., Paradis, M.V., Couture,P. Study of the acute impact of polyphenols from brown seaweeds on glucose control in healthy men and women. FASEB J.2010;24: Meeting abstract supplement No. 209.4.
- Syiem, D., Syngai, G., Khup, P.Z., Khongwir, B.S., Kharbuli, B., Kayang,H. Hypoglycemic effects of Potentillafulgens in normal and alloxan induced diabetic mice. J. Ethnopharmacol.,2002; 83: 55-61.
- Ranilla, L.G., Kwon, Y.I., Apostolidis, E., Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol.,2010; 101(12): 4676-89








