Zabihollah Khaksar*, Sara Azhdari and Mahnaz Taherianfard
Department of Basic sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
DOI : https://dx.doi.org/10.13005/bpj/921
Abstract
Parkinson’s disease is a degenerative disease of extrapyramidal system. After Alzheimer’s disease, it is placed at the second category of degenerative diseases. The core cells of substantianigra located in midbrain is gradually disappeared and dopamine receptors and GABA neurons are reduced in the brain, in Parkinson’s disease. It is likely that the disease has genetic backgrounds. Since chemical medicines have multiple complications on rats’ fetuses and offsprings from mother with Parkinson’s disease, so the purpose of this study is histomorphometric investigation of lycopene’s effect on neurons containingdopamine receptors and GABA in Offsprings rat from mothers with Parkinson’s disease. A total number of 40 adult female rats were used in this study and randomly divided into five groups, including: control group, patient group (6-hydroxydopamine was unilaterally injected into right side ofsubstantianigra by Hamilitonsyringe), sham group (0.02% ascorbic acid was unilaterally injected intoright side ofsubstantianigra), lycopene control group (received 0.5 ml/kg lycopene by gavage), and lycopene treatment group (induction of Parkinson’s disease + 0.5 ml/kg lycopene). After 15th and 30th days of the experiment, the pups were anesthetized and their brains removed for the rest of the processes. The density of neurons containing D1, D2 dopamine and GABA receptors were then analyzed with immunohistochemistry method using optical microscope. The obtained results showed reduction in the number of neurons containing dopamine receptors (D1, D2) and GABAin both hippocampus and amygdala regions related to 15- and 30-day old pups of the patient group. The number of neurons containing D1, D2 dopamine and GABAreceptors in hippocampus and amygdala regionsof the lycopene treatment group increased compared tothose of the patient group. Probably due to the production of free radicals and lipids peroxidation, 6-hydroxydopamine in a time-dependent manner reduces neurons containing D1 and D2 dopamine receptors and GABA in hippocampus and amygdala regions. Reduction of receptors in amygdala region is more than the hippocampus area. Because of neuroprotective and antioxidant properties, lycopene extract increases these neurons and thereby improves Parkinson’s disease in under treatment group.
Keywords
Histomorphometric; Lycopene; Dopamine Neurons; GABA Neurons; Parkinson’s disease; Offspring Rats
Download this article as:| Copy the following to cite this article: Khaksar Z, Azhdari S, Taherianfard M. Histomorphometric Study of Lycopene Effect on the Number of Neurons Containing D1, D2 and GABA Receptors in Hippocampus and Amygdala in OffspringsRat from Parkinson’s Mothers. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Khaksar Z, Azhdari S, Taherianfard M. Histomorphometric Study of Lycopene Effect on the Number of Neurons Containing D1, D2 and GABA Receptors in Hippocampus and Amygdala in OffspringsRat from Parkinson’s Mothers. Biomed Pharmacol J 2016;9(1). Available from: http://biomedpharmajournal.org/?p=6567 |
Introduction
Parkinson’s disease is the second degenerative disorder of extrapyramidal system after Alzheimer’s disease. The core cells of substantianigra located in midbrain is gradually disappeared and therefore levels of dopamineproduced in the brain by these areasare reduced in the Parkinson’s disease [1]. The disease occurs in all races. The prevalence of 1-2 case(s) per thousand people with almost equal gender distribution has been reported in the United States and Western Europe. The disorder is more common with increasing age [2].
It was stated about the mechanism of Parkinson’s disease that it occurs due to the loss of secreting cells of a substance called dopamine [3, 4]. Evidences suggest that dopamine has dual capability in pathogenesis or medicinal treatment of central nervous system disorders such as Parkinson’s and schizophrenia diseases [5]. It is also stated that the main motor difficulties arises in Parkinson’s disease are created by functional defect in nigrostriatal dopaminergic system [6]. Bhargave in 1990 announced that dopamine has an important role in prevalence of psychotic behaviors. There are also some reports suggesting that dopamine plays an important role in some motor and behavioral disorders. Studies expressed that some factors such as oxidative stress, increased lipid peroxidation, reduced glutathione levels, DNA destruction, and iron accumulation are the major causes of dopaminergic neurons degeneration [7].
On the other hand, some investigations on neuropathic animals showed lack of change in GABA receptors, GABAergicinterneurons, their synthesizing enzymes, and GABA levels [8]. Other researches also reported peripheral nerve injury followed by an increase in GABA levels in the spinal cord dorsal horn and even increase of inhibitory effect in the neurons of this area [8]. This represents the relationship between dopamine and GABAergic systems with some neurological diseases such as Parkinson’s disease.
Many studies were performed by the aim to determine the inhibitory effect of dietary antioxidant on Parkinson’s disease [9]. Among dietary antioxidants that many researches have performed on them can mention to vitamin E, vitamin C, and Carotenoids (beta-carotene), which protect cells against oxidative damages [10]. A study in 2007 also reported that oral administration of ginseng extract stops cell destruction in substantianigra and decreases motor disorders in mice with Parkinson’s disease [11].
Lycopene is also a dietary carotenoid produced by plants and microorganisms. This pigment mainly exists in fruits with red color, vegetables, tomatoes, watermelon, red grapes and apricot [12]. It has been received a lot of attention and many studies have been performed on it due to its antioxidant properties. Presence of a large number of double bonds in lycopene structure makes it one of the most effective carotenoids that can neutralize oxygen radicals [13]. Given to the antioxidant properties of lycopene, significant scientific studies and extensive clinical researches have been assignedtolycopene consumption and general health. Recent researches point to some improvements in cardiovascular diseases, cancer, diabetes, osteoporosis, as well as infertility in men [14]. The antioxidant activity of lycopene has been widely studied and it has been shown that lycopene is able to neutralize free radicals in cell culture and animal models [15].
Considering the above mentioned issues and the possible role of free radicals in incidence of Parkinson’s disease and its unknown adverse effects on infants born to mothers with Parkinson’s disease, as well as side effects caused by chemical medications in pregnant mothers and in their fetuses and infants, this study tries to investigate antioxidant properties of lycopene on improvement of neurons containing dopamine receptors and GABA in rat pups born to mothers with Parkinson’s disease to considerably help tothe disease improvement.
Methods
A total number of 40 adult female Sprague-Dawley rats aged of 3-3.5 months, weighing 20020g were used in this study. For adaptation to condition and before beginning of the research, all animals were kept in standard Plexiglas cages for 2 weeks. They were provided with food and water, freely. The rats were kept in the controlled temperature (222), and a cycle of 12 hours of light and 12 hours of darkness.
The rats were studied in 5 groups of 8 each:
Control group: it was consisted of 8healthy rats, without any prescription.
Lycopene control group: it was consisted of 8 healthy rats. They daily received 0.5 ml/kg BW lycopene dissolved in 0.5% sodium carboxy-methyl-cellulose by gavage, until the time of parturition [16].
Sham group: it was consisted of 8rats. This group is similar to the patient group, but the rats in this group only were injected 0.02% ascorbic acid in 0.9% solution of sodium chloride (saline) to their right side of substantianigra.
Patient group: it was consisted of 8rats. Parkinson’s disease was induced in the rats by unilaterally injection of 6-hydroxydopamine into right side of substantianigra [17].
Lycopene treatment group: it was consisted of 8 rats with Parkinson’s disease. This group, similar to the group 2, received 0.5 ml/kg BW lycopene until the parturition time.
The method of induction of Parkinson’s disease will explain, as the following: for this purpose, the rats were anesthetized with ketamine (87 mg/kg) + xylazine (13 mg/kg) [13] and 6-hydroxydopamine (5 μg) was solved in 0.02% ascorbic acid in 0.9% sodium chloride (saline) solution. The obtained solution received to 2μl volume and then was injected (at a rate of 0.5 μl/min) with a Hamilton syringe and according to the rat Brain Atlas (Paxinos) into the right side of the brain substantianigra [17, 18].
After getting used to the environment (all groups) and consolidation of Parkinson’s disease, two females were placed with a male rat in separate cages for mating. After mating and observation of vaginal plug, the first day of pregnancy was determined. After birth, the newborn rat pups were anesthetized with thiopental sodium (Nesdonal) at 15th and 30th days of the experiment. Their skulls immediately were opened and the entire brains removed. They were then fixed in 4% buffered formalinsolution. The samples were then transferred to autotechnicon device. After passing through various stages of passage including dehydration, transparency, and embedding in paraffin in the device, the samples were molded and coronal brain sections (5-6 μm thickness) were prepared as a serial using a microtome. The obtained sections were stained using immunohistochemistry methods and histomorphometric studies were carried out on amygdala and hippocampus regions. The obtained results were analyzed using SPSS version 18.
Results
According to Chart (1), the number of neurons containing D1, D2, and GABA receptors in the patient group shows a significant decrease compared to the control group (p<0.05)in amygdala region in 15-day old of offspring rats. Also the number of neurons containing D1, D2 and GABA receptors in the lycopene treatment group were increased compared to those of the patient group, which is not significant.The number of neurons containing D1, D2, and GABA receptors in the lycopene treatment group were increasedcompared to those of the control group, which is not significant (p<0.05).
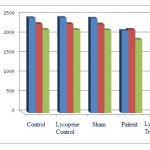 |
Figure 1: The average number of neurons containing D1, D2, and GABA receptors in amygdala region in 15-day old of offspring rats. |
According to Chart (2), the number of neurons containing D1, D2, and GABA receptors in the patient group shows a significant decrease compared to those of the control group at (p<0.05)level, which shows negative effects of Parkinson’s disease on the number of neurons containing D1, D2, and GABA receptors in hippocampus region in15-day old of offspring rats. Also the number of neurons containing D1, D2 and GABA receptors in the lycopene treatment group were increased compared to those of the patient group. This is not statistically significant at (p<0.05) level, but shows positive effects of lycopene on improvement of Parkinson’s disease complications, so that, the number of neurons containing D1, D2 and GABA receptors in the lycopene control group were increasedcompared to those of the control group, which is not statistically significant at (p<0.05) level.
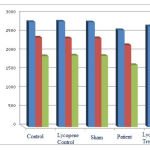 |
Figure 2: The average number of neurons containing D1, D2, and GABA receptors in hippocampus region in 15-day old of offspring rats. |
According to Chart (3), the number of neurons containing D1, D2, and GABA receptors in the patient group shows a significant decrease compared to the control and the sham groups at (p<0.05) level, which indicates negative effects of Parkinson’s disease on the number of neurons containing D1, D2, and GABA receptors in amygdala region of 30-day old of offspring rats. On the other hand, an increase in the number of neurons containing D1, D2, and GABA receptors in the treatment with lycopene group and in the lycopene control groupis observed compared to the patient and control groups, respectively. This is not statistically significant at (p<0.05) level. However the increase in the number of neurons indicates positive effects of lycopene on changes of receptors compared to the patient group.
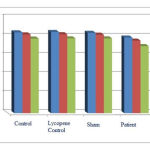 |
Figure 3: The average number of neurons containing D1, D2, and GABA receptors in amygdala region in 30-day old of offspring rats. |
According to Chart (4), the number of neurons containing D1, D2, and GABA receptors in the patient group shows a significant decrease compared to those of the control group at (p<0.05) level. This result indicates that the receptors are decreased by Parkinson’s disease in hippocampus region in 30-day old of offspring rats. The number of neurons containing D1, D2, and GABA receptors in the lycopene treatment group and in the lycopene control group shows a significant increase compared to those of the patient group and the control group, respectively. This result indicates positive effects of the extract on hippocampus region of 30-day old rat pups with Parkinson.
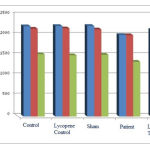 |
Figure 4: The average number of neurons containing D1, D2, and GABA receptors in hippocampus region in 30-day old of offspring rats. |
Discussion
The results show that 6-hydroxydopamine-induced Parkinson’s disease in rats decreases neurons containing D1, D2, and GABA receptors in hippocampus and amygdala regions in the groups in which the disease induced. This shows destruction side effects of 6-hydroxydopamine.
Studies suggested that free radicals are chemical compounds with high reactivity properties. These compounds can cause mutations, lipids peroxidation and proteins oxidation [19]. According to the researches, injection of 6-hydroxydopamine causes oxidative stress, destruction of membranes and proteins, as well as destruction of dopamine receptors in the brain and thereby leads to induction of Parkinson’s disease in the brain [20]. 6-hydroxydopamine based on the researches can lead to cell death in nerve cells and resulting in Parkinson’s disease in rats [21]. Some studies showed that there is a possible link between the disease, oxidative stress and lipids peroxidation [22].
Studies also reported that 6-hydroxydopamine as a neuroleptic medicine is able to block D2 receptors and through that causes destruction and disorder in nigrostriatal pathway and in many other agents, such as GABA receptors [21]. It is also revealed that 6-hydroxydopamine is an oxidative neurotoxin which destructs dopaminergic neurons in vivo and in vitro conditions [23]. The possibility exists that dopamine receptors and GABA neurons are dispersed in other parts of the brain especially in hippocampus and amygdala areas. Studies suggested that there is a relationship between the rest of the brain, especially between midbrain and hippocampus and substantianigra [24]; and disturbance in a brain pathway also causes disturbance in hippocampus and amygdala regions. On the other hand, it was stated in studies that midbrain dopaminergic neurons are destroyed in Parkinson’s disease and reduce dopamine in the straitum body and hippocampus and after that motor, memory, and learning disorders create in patients [25]. In researches done in the past on Parkinson’s diseases expressed that reduced level of dopamine will decrease neurogenesis in dentate gyrus and synapses between neurons in hippocampus area. This reveals that a disorder in a point of the brain in Parkinson’s disease leads to progress in disorders in other points [26]. This agrees with the results of this study. As previously mentioned, probably in this study reduction of dopamine receptors and GABA neurons will be due to the injection of 6-hydroxydopamine, induction of oxidative stress, and death of nerve cells, which is in consistent with previous researches.
Studies revealed that the density of dopamine receptors and GABA neurons that are related to each other [27] have different densities in various diseases. As in some diseases such as epilepsy, the density of GABA neurons in hippocampus area shows a greater reduction [28-30]. This agrees with the result of this study. The results also show that the density of receptors in hippocampus area is more than amygdala region [31].
According to the results of the present study, an increase in the number of neurons containing dopamine receptors and GABA is observed in the groups received lycopene. This shows the positive effects of lycopene extract. Today, low molecular weight antioxidants (e.g. vitamin E and C) and large protein molecules (e.g. superoxide dismutase, glutathione peroxidase, and reduced glutathione) are used in treatment of many diseases related to the brain damage caused by oxidative stress such as Parkinson’s disease [32]. Many researches are performed by the aim to determine inhibitory effect of dietary antioxidants on the disease [33]. Lycopene is a strong antioxidant that reduces oxidative damage to DNA and thereby decreases the cancer affliction risk. In a study in 2007 reported that oral administration of herbal extracts including lycopene and antioxidant ingredients leads to stop in cell destruction. It is also reduced motor disorders in mice with Parkinson’s disease [11]. Studies about the antioxidant and neuroprotective properties of lycopene extract showed that this substance is effective in the treatment of neurodegenerative diseases. This is due to its antioxidant properties in the treatment of diseases [15], which is consistent with this study results. As, a time-dependant improvement in the number of dopamine receptors and GABA neurons is observed in the groups received lycopene.
Conclusion
Given to the above, 6-hydroxydopamine probably due to the production of free radicals and also lipids peroxidation in a time-dependent manner reduces the number of neurons containing dopamine receptors and GABA in hippocampus and amygdala regions. Lycopene extract, due to its antioxidant and neuroprotective properties, increases the number of neurons containing receptors and thereby improves Parkinson’s disease in under treatment groups. Therefore, the use of lycopene extract is recommended for reduced complications in the disease and the results may be somewhat extended to human.
References
- Chuang C, Su H, Cheng F, Hsu SH, Chuang CF, Liu CS. Quantitative evaluation of motor function before and after engraftment of dopaminergic neurons in a rat model of Parkinson’s disease. J Bio Sci. 2010 Feb; 17(13):9.
- Roger P, Simon M, David A. Greenberg R, Aminoff MJ. Movement disorders. Clinical Nreurology. 7th ed. McGrawHill, 2009, 238-269.
- Tadibi V. Yosefi B. Taheri HR. Taherzadeh J, Taherzadeh M. The impact of a physical therapy regimen on motor function in people with parkinson disease. World J Sport Sci. 2008; 1(1): 48-53.
- Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, et al. Running exercise protects the substantianigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011; 25(1): 135-46.
- Strange PG. The binding of agonists and antagonists todopamine receptors. BiochemSoc Trans 1996; 24(1):188-92.
- Buob A, Winter H, Kinderman M, Becker G, Möller JC, Oertel, WH, et al. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson’s disease. Clin Res Cardiol. 2010; 99(11): 701–6.
- Schwarting RK, Huston JP. Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology 1997; 18: 689-708.
- Lee M, Schwab C, Mcgeer PL. 2010. Astrocytes are GABAergic cells that modulate microglial activity. Neuroscience, 59 (1): 152-165.
- Olanow CW. Dietary vitamin E and Parkinson’s disease: something to chew on. Lancet Neurol 2003; 2:74.
- Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins: vitamins E and C, beta carotene, and other carotenoids. Ann NY Acad Sci. 1992; 669:7-20.
- Lopez MV, Cuadrado MP, Ruiz-Poveda OM, Del Fresno AM, Accame ME. Neuroprotective effect of individual ginsenosides on astrocytes primary culture. BiochimBiophysActa. 2007; 1770(9): 1308-16.
- Holic CN, Giovannucci EL, Ronser B, Stampfer MJ, Michaud DS. Prospective study of intake of fruits, vegatables and carotenoids and the risk of adult glioma. Am J Nutr. 2007; 85(3): 864 77. [PMID= 17344512]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the united states. J Agric Food Chem. 2004; 52(12): 4026-37. [PMID: 15186133]
- Giovannucci E, Willett WC, Stampfer MJ, Liu Y, Rimm EB ,2002. “A prospective study of tomato products, lycopene, and prostate cancer risk”. J. Natl Cancer Inst 94 (5): 391–396.
- Cohen LA. A reviw of animal model studies of tomato carotenoids, lycopene and cancer chemoprevention. ExpBiol Med. 2002; 227: 864-8. [PMID:12424327]
- Kumar P, Kalonia H, Kumar A. Lycopene modulates nitric oxide pathways against 3-nitropropionic acid-induced neurotoxicity. Life sciences. 2009;85(19):711-8.
- Jin F, Wu Q, Lu Y-F, Gong Q-H, Shi J-S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. European journal of pharmacology. 2008;600(1):78-82.
- DABBENI-SALA F, DI SANTO S, FRANCESCHINI D, SKAPER SD, GIUSTI P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. The FASEB Journal. 2001;15(1):164-70
- Sanyal J, Bavdyopadhyay SK, Banerjee TK, Mukherjee SC, Chakraborty DP, Ray BC, Rao VR. Plasma levels of lipid peroxides in patient with Parkinson`s disease. Eur Rev Med PharmacolScie 2009; 13: 129-132.
- Aligholi H, Sarkaki A, Badvi M et al., 2011. Effects of pretreatment with various doses of soy isoflavones on learning process and spatial memory in overiectomized rat model of Parkinson’s disease. Iran Ninth Congress of Nutrition, Tabriz University of Medical Science and Health Services.
- Rayhani Rad S, Mahmoudi J. 2015. The influence of A2A adenosine receptors on Katalpsy caused by 6-hydroxydopamine in rats. Scientific-Research Comparative Pathophysiology.
- Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL. Adult nutrient intake as a risk factor for Parkinson’s disease. Int J Epidemiol. 1999; 28:1102-9.
- Blum K, Panayotis KT, Mark SG and et al. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014; 5: 919.
- Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. BehavSci Law. 2008;26(1):65-83.
- Bernheimer H, Birkmayer W, Hornykiewicz O and et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415-55.
- Martig AK, Sheri J, Mizumori Y. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus , Vol 21 Issue 2 : 172- 184.
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren J-Q, Bhide PG. Dopamine receptor activation modulates GABAergic neuron migration from the basal forebrain to the cerebral cortex. The Journal of neuroscience. 2007;27(14):3813-22.
- Tian M, Macdonald RL. The intronic GABRG2 mutation, IVS6+2T->G, associated with childhood absence epilepsy altered subunit mRNA intron splicing, activated nonsense-mediated decay, and produced a stable truncated gamma2 subunit. J Neurosci. 2012; 32: 5937-52.
- Reid CA, Kullmann DM. GABAA receptor mutations in epilepsy (commentary on Lachance-Touchette et al.). Eur J Neurosci. 2011; 34: 235-6.
- Drexel M, Kirchmair E, Sperk G. Changes in the expression of GABAA receptor subunit mRNAs in parahippocampal areas after kainic acid induced seizures. Front Neural Circuits. 2013; 7: 1-13.
- Charuchinda C, Supavilai P, Karobath M and et al. Dopamine D, Receptors in the Rat Brain: Autoradiographic Visualization Using a High-Affinity Selective Agonist Ligand. The Journal of Neuroscience, 1987; 7 (6): 1352- 1360.
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidants therapy in Parkinson’s disease. Prong Neurobiol 1996; 48: 1-19.
- Olanow CW. Dietary vitamin E and Parkinson’s disease: something to chew on. Lancet Neurol 2003; 2:74.







