P. R. Janani, A. Murugan1, and S. T. Santhiya2
1Department of Microbiology, Periyar University, Periyar Palkalai Nagar, Salem-636011, 2Department of Genetics, A.L.Mudaliar P.G.IBMS, University of Madras, Taramani, Chennai – 600113.
DOI : https://dx.doi.org/10.13005/bpj/946
Abstract
Spontaneous abortion in the first trimester of pregnancy is problem among Indian women accounting for 15 to 20 percent pregnancy loss. Couples with abortion histories are probably at greater risk for cytogenetic abnormality than the couples with normal children. The abnormality associated with recurrent pregnancy loss includes translocation, inversions, recurrent aneuploidy, marker or supernumerary chromosomes, heterochromatic polymorphic etc. Hence, the present study is focused to assess the prevalence of chromosomal abnormalities among couples with repeated abortion cases. It was extrapolated that repeated pregnancy loss has was very common with abnormality in the 22p(stk+) in male.
Keywords
Spontaneous abortions; translocation; heterochromatic polymorphic
Download this article as:| Copy the following to cite this article: Janani P. R, Murugan A, Santhiya S. T. Chromosomes Abnormalities Among Recurrent Spontaneous Abortions Cases. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Janani P. R, Murugan A, Santhiya S. T. Chromosomes Abnormalities Among Recurrent Spontaneous Abortions Cases. Biomed Pharmacol J 2015;9(1). Available from: http://biomedpharmajournal.org/?p=6501 |
Introduction
Recurrent spontaneous abortion (RSA) has been associated with multiple factors. However, etiologies of the RSA remain unclear for a longer period and due multifactorial incidences it is often misleading in the diagnosis and treatment. It is learned that RSA affects about 1% of child bearing population [1]. Reasonably accepted etiologic causes including genetics, anatomical, placental anomalies, hormonal problems, infection, hereditary thrombophilia, immunologic factors, nutritional and environmental factors are reported at different ratios [2].
Chromosomal abnormalities in the conceptus are usually the characteristic findings in cases of spontaneous abortion due to problems with the Chromosomal heteromorphism is considered a variant of a normal karyotype, but it is more frequent in couples with RSA [3]. At the first time, Carr (1960) reported that about 50% of first trimester abortions are due to structural or numerical chromosome abnormalities [4]. Evidence suggests that 12% of the patients with the history of recurrent miscarriages have chromosomal abnormalities [5]. In a study done in India Chromosomal aberrations were found in 8.57% of patients in which Numerical abnormalities 0.95%, Structural abnormalities 2.87% and polymorphic variants were 4.76%.This shows that cytogenetic analysis can be considered while exploring the cause of recurrent pregnancy loss [6].
Somatic and/or germ cell chromosomal abnormalities, deletions of the azoospermia factor regions in the proximal long arm of Y chromosome, DNA damage in spermatozoa and single gene mutations constitute the genetic component. Chromosomal abnormalities have been recognized to occur in infertile men with azoospermia/oligozoospermia and/or abnormalities involving sperm morphology/ motility. These anomalies may be numerical or structural and involve the sex chromosomes or auto somes [7]. Progress during the past few years has shown that infertile men have an 8–10-fold higher prevalence of chromosomal abnormalities than fertile men [8]. Autosomal polymorphisms mainly indicate mutations of the heterochromatin distributed in the centromere, telomere, or satellite and at secondary constrictions. Chromosomal abnormality on chromosomes 1, 9, 12 and 22. Autosomal polymorphisms (45.44 %, 10/22) were associated with habitual abortion (45.44 %), AS (18 %) and stillbirth (18 %) [9].
Robertsonian translocation is the frequently seen autosomal CA in infertile men (0.7%). A correlation has been observed between the XY bivalent and the chromosomes involved in Robertsonian trivalent in the pachytene stage of meiosis leading to the impairment of the germ cells [10]. Pooling the data from the different series of infertile males, 0.5% reciprocal translocations were observed as compared to 0.1% in the newborn children. The correlation between reciprocal translocations with involvement of chromosomes 3, 4, 5, 6, 7, 9, 11, 13, 14, 15, 16, 17, 19, 20, 21, 22 and the impairment of sperm production is well known [11].
In this study, we present the cytogenetic results with proven infertility. Associations between the phenotypes of the individuals and chromosomal abnormalities were investigated. The results could help with the development of a more efficient screening program to benefit prospective patients, and provide information to assist with genetic counseling and the selection of more appropriate therapies.
Materials and Methods
It is conceivable that a specific family/genetic component may play a role in the aeitology of abortion, even when clinically evident genetic anomalies have been ruled out by the determination of parental karyotypes from peripheral leukocytes.
Samples
The present study includes couples with bad obstetric history of N=6 couples were referred to Dr.ALMPG IBMS Chennai, Dept of Genetics for cytogenetic diagnosis were chosen for the present study. The case history of the patients were analyzed by means of Pedigree chart.
Chemicals
RPMI 1640 tissue culture medium, fetal bovine serum, Phytohemagglutinin, Colchicine, Potassium chloride, Methanol and Glacial Acetic Acid.
Preparation
Medium Preparation
10.3g of RPMI 1640 medium powder was dissolved in a liter of autoclaved distilled water. The solution was then filtered and the antibiotics such as 1ml of Benzyl Penicillin [5000 IU Benzyl Penicillin was dissolved in 5ml of sterile water] and 0.4ml Ambistryn –S [0.75g Ambistryn –S was dissolved in 3ml of sterile water]. Then the PH of the medium was checked (7.2-7.5).
Colchicine (0.01%)
5mg of colchicine was added to 50ml of double distilled water.
Hypotonic solution (0.75M)
559mg of Potassium Chloride was added to 100ml of distilled water and maintained at 37ºCelsius.
Giemsa stain preparation
10% buffer solution: 5gms of Disodium Hydrogen Phosphate salt was added in 50ml of double distilled water.
Giemsa working solution
From Giemsa stock solution 2ml was added to 2ml of methanol solution and 46ml of distilled water was added to make it up to 50ml.
Trypsin (0.25%)
0.25gms of trypsin was dissolved in 50ml of double distilled water.
Methodology
Culture
Peripheral blood leukocyte culture was done by following the procedure of Hungerford (1965). 2ml of venous blood was drawn into a sterile heparinized syringe of which 0.5ml was added to sterile culture vial containing 5ml of culture medium. 1ml (20%) of fetal bovine serum and 0.2ml of phytohemagglutinin (PHA). Then the contents was mixed gently and incubated at 37ºC for 3 days.
Harvesting
At the end of 3days, 1 drop of colchicine was added to the cultures to induce mitotic arrest and incubated for 30mins. Then the culture was transferred to the centrifuge tubes and centrifuged at 1000rpm for 5mins. The supernatant was discarded and hypotonic solution was added to make into 6ml and vortexed gently and incubated for 15mins. After incubation add 1ml of carnoy’s fixative (3:1) methanol and acetic acid was added and tubes were centrifuged at 1000rpm for 5mins. The supernatant was discarded and freshly prepared fixative was added and left at room temperature for one hour. The supernatant was discarded and up to 6ml of freshly prepared fixative was added and kept under refrigeration for 24 hours, prior to the slide preparation.
Slide Preparation
The cell suspension was centrifuged at 1000rpm for 5 mins and the supernatant was discarded. Few drops of fixative were added to make a uniform suspension. 4 drops of cell suspension was dropped onto a prechilled clean slides and blown gently to obtain an even spread. The slide was immediately placed over a slide warmer maintained at 40ºCelsius. The slide was then observed under microscope to check the mitotic index and spread of chromosomes. The preparation of other slides was done accordingly.
Karyotyping
For further chromosomal analysis GTG-banding (Giemsa banding) was done. The prepared slides were treated in 0.5% trypsin for 7-10 sec. Treatment with trypsin was altered each time depending upon the age of the slides. Then the slides were rinsed in double distilled water and stained in 4% buffered Giemsa solution for 5mins. They were rinsed in double distilled water, air dried and checked under the microscope for quality of banding and staining intensity.
Chromosome Analysis & photomicrography
20 well spread and well banded metaphases were analyzed under oil immersion (100x) objective, (NIKON, E200 JAPAN) at 450 BPHS (Bands per Haploid Set). Well banded representative metaphases of each case were photographed (NIKON PHOTOMICROSCOPE E200, MODEL, JAPAN) and karyotyped.
Results
Cytogenetic analysis was done for cases referred for recurrent spontaneous abortions, to the Dept of Genetics, Institute of Obstetrics & Gynecology Egmore, Chennai and Dept of Genetics, Dr. ALM PG IBMS, University of Madras, Chennai.
Twenty cases well GTG-banded complete metaphase spreads were analyzed under oil immersion (100 X) objective to rule out the presence of constitutional chromosome abnormalities. Of these 6 cases has normal karyotype as 46XX, 46XY with other complications . But one case has 22p stk+.
Case History Of The Patients
Case No: 1
Wife’s TORCH test, thyroid tests are normal. Sperm analysis revealed that husband had Asthenospermia. The second child had trisomy of 69XXY.
Case No: 2
Second and third fetus with Neural Tubal Defects (NTD). The wife has dysgerminoma in right ovary.
Case No: 3
The wife has irregular mensutral cycle since menarche.
Case No: 4
Wife’s TORCH test showed positive for Rubella IgG and CMV IgG (90.4 IU/ml and 84.9AU/ml).
Case No: 5
Wife’s TORCH test showed positive for Rubella IgG and CMV IgG (26.3 IU/ml and >250.0 AU/ml).
Case No: 6
Still born male child and terminated at 4 months due to lack of fetal heart beat.
Pedigree Chart of the Cases
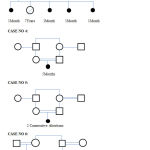 |
Figure 1 |
Table 1: Case sheet of RSA patients
| SNo | Case No | Age/Sex | C/NC | No of Abortions | Period of abortions | TORCH TEST | KARYOTYPE RESULTS | |||
| Age | Sex | 1ST | 2ND | SB | ||||||
| 1. | BOH 81 | 31 | M | NC | 2 | 2 | – | – | -VE | 22 pstk 46XY |
| 27 | F | NORMAL 46 XX | ||||||||
| 2. | BOH 79 | 38 | M | NC | 3 | 1 | – | 2 | NI | NORMAL 46XY |
| 33 | F | NORMAL 46XX | ||||||||
| 3. | BOH 67 | 40 | M | NC | 4 | 4 | NI | NORMAL 46XY | ||
| 35 | F | NORMAL 46XX | ||||||||
| 4. | BOH 75 | 40 | M | C | 1 | 1 | RUB,CMV +VE | NORMAL 46XY | ||
| 31 | F | NORMAL 46XX | ||||||||
| 5. | BOH 78 | 28 | M | C | 1 | 1 | RUB,CMV +VE | NORMAL 46XY | ||
| 23 | F | NORMAL 46XX | ||||||||
| 6. | BOH 41 | 37 | M | C | 4 | 3 | 1 | NI | NORMAL 46XY | |
| 30 | F | NORMAL 46XX | ||||||||
NC= Non Consanguineous SB=Still Born C= Consanguineous F=Female
NI=Not Identified M=Male
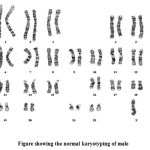 |
Figure 2 |
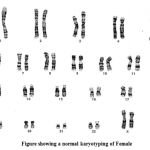 |
Figure 3 |
 |
Figure 4 |
Discussion
One of the most frequent reproductive events is recurrent spontaneous abortion (RSA) that occurs in women in reproductive age with a frequency of 1–3 % [12]. The contribution of polymorphic chromosomal variants to infertility is still questionable and future investigations including those using microarray and gene expression profiling technologies on larger study populations are required to delineate the role of the apparently “harmless” chromosomal polymorphisms.
If there is history of previous abortions, it is likely that chromosomal aberrations will be found with greater frequency in them. Various chromosomal abnormalities like reciprocal translocation, centric fusion and mosaicism have been reported in cases with recurrent abortions. Therefore, in contrast to the current opinion, the polymorphism is acrocentric chromosomes seems to have some functional effects; however, probably due to a gene-dose-compensation mechanism, they are without clinical relevance [13].
Qualitative polymorphisms included those located in the centromeric regions of chromosomes 3 and 4 (3c, 4c) and on the short arms and satellites of chromosomes 13, 14, 15, 21, and 22 (13p, 13s, 14p, 14s, 15p, 15s, 21p, 21s, 22p, 22s). These polymorphisms were designated as present or absent on each of the homologs in question [14].
When a Robertsonian translocation causing Down Syndrome (DS) is inherited, the recurrence risk in the future pregnancy depends on the parental sex. In presence of paternal carrier for t (21:22) the recurrence risk if 5%. It increases further to 10% if mother is a carrier. Over two-thirds of the Down Syndrome (DS) conceptions miscarry spontaneously or are stillborn. A number of tests including screening tests for DS have been devised over last few decades for the prenatal diagnosis of various genetic diseases [15].
The t(21;22) is the only known recurrent, non Robertsonian constitutional translocation. The majority of t(11;22) translocations occur within the same genomic intervals and that the majority of supernumerary-der (22) offspring result from a 3:1 meiosis I malsegregation in the balanced-translocation carrier [16].
The structural anomalies were 40 translocations and 9 duplication/ deletion/ marker/ iso chromosome for the X chromosome; Male: 46,XY/ 47,XY+ mar (1); Female: 45,X/ 47,XX+mar (1); 46,XX/ 47,XX+mar (1); 47,XX+frag (1); 46,X,Xq- (2); 46,X,Xp- (1); 46,X,Xp+ (1); 45,X/46,X,i(Xq)(1). The frequently involved chromosomes in the translocations were 4, 11, 15 and X. There were three X; autosomal translocations and a unique combination of translocation 1; 15 in the parents of a female carrier and 13; 14 in a non- consanguineous couple. On the whole, 57.5% of the females (23/ 40) were translocation carriers. Non-significant chromosome polymorphisms were observed in 79 cases (4.2%) [17].
Most infertile men exhibited reduced levels of testosterone. Patients with Robertsonian and sex chromosome–autosome reciprocal translocations were reported to have significantly lowered testosterone levels when compared with those who had an autosome-autosome translocation and the fertile control group [18].
It can be extrabulated from the present study that spontaneous abortion may be due to chromosomal abnormality. In this study CASE NO: 1 the abortion may be due to chromosomes abnormality. The presence of stalk has been proved that this may be the main cause for spontaneous abortion but it may also be the cause. Many studies are going on that the stalk may lead to spontaneous abortion. The presence of stalk may lead to Turner’s syndrome, Down’s syndrome and Klinefelter syndrome. These are all due to non-disjunction which may involve in meiosis. An increase in the length of heterochromatic regions namely 1qh+, 9qh+, 15p+, 21p+, 22p+ and Yqh+ were noted in azoospermia and oligoasthenoteratozoospermia groups of infertile men and the pericentric inversion inv(9qh) was noted in one case of azoospermia [19].
Conclusion
Thus in this study it is shown that chromosome plays a main part in the spontaneous abortion. The chromosomal analysis is kept as the last test to rule out the chromosomal abnormality. It is shown that the 22p (stk+) the cause for the spontaneous abortion. recent studies by cell biologists suggest that heterochromatin may have important cellular roles in different clinical conditions, including fertility
Acknowledgement
This work was carried out with the support of Dr.A.L.Mudaliar Post Graduate Institute of Basic Medical Sciences, Taramani, Chennai – 600113. Thanks are extended to my professors and my seniors to carry out this work.
References
- Stirrat GM: Recurrent miscarriage. II: clinical associations, causes, and management. Lancet 1990; 336:728–733. 2 Branch DW, Gibson M, Silver RM: Clinical practice. Recurrent miscarriage. N Engl J Med 2010; 363:1740–1747.
- Tang, A. W., and Quenby, S.(2010).Recent thoughts on 3. Management and prevention of recurrent early pregnancy loss. Curr. Opin. Obstet. Gynecol. 22, 446–451.
- Caglayan AO, Ozyazgan I, Demiryilmaz F and Ozgun MT 4. (2010). Are heterochromatin polymorphisms associated with recurrent miscarriage? J. Obstet. Gynaecol. Res. 36: 774-776.
- Dewhurst J. Fertility in 47, XXX and 45, X patients. J Med Genet 1978; 15: 132-135.
- Hassold T, Chiu D. Maternal age specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 1985; 70: 11-17.
- Rajasekhar, P. M. Gopinath, K. Sreelakshmi and K. Satyamoorthy. A Cytogenetic Study of Couples with Miscarriages: An experience int J Hum Genet. 2013; 13(2): 93-97.
- Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Tournaye H, et al. Guidelines on Male Infertility. European Association of Urology, 2013; 14-20.
- McLachlan RI, O’Bryan MK. State of the art for genetic testing of infertile men. J Clin Endocrinol Metab. 2010; 95(3):1013–24.
- Dingyang Li & Hongguo Zhang & Ruixue Wang & Haibo Zhu & Linlin Li & Ruizhi Liu. Chromosomal abnormalities in men with pregestational and gestational infertility in northeast China. J Assist Reprod Genet (2012) 29:829–836.
- Johannisson R, Schwinger E, Wolff HH, vom Ende V, Lohrs U. The effect of 13; 14 Robertsonian translocation on germcell differentiation in fertile males. Cytogenet Cell Genet., 1993; 63: 151-155.
- Gabriel–Robez O, Rumpler Y. The meiotic pairing behavior in human spermatocytes carrier of chromosome anomalies and their repercussions on reproductive fitness. II Robertsonian and reciprocal translocations. A European collaborative study. Ann Genet., 1996; 39: 17-25.
- T., et al,.(2013) Stul polymorphism on the an1. drogen receptor gene is associated with recurrent spontaneous abortion .JAssist Reprod Genet 30:437-440.
- F. Zakharov , A. Z. Davudov , V. A. Benjush , N. A. Egolina. Polymorphisms of Ag-stained nucleolar organizer regions in man. Original Investigations Human Genetics., 1982; 60 (4): 334-339.
- Bruce D. Blumberg, Jeffrey D. Shulkin, Jerome I. Rotter, Thuluvancheri Mohandas, and Michael M. Kaback. Minor Chromosomal Variants and Major Chromosomal Anomalies in Couples with Recurrent Abortion. Am J Hum Genet, 1982; 34:948-960.
- Verma I.C, Mathew .S, Elango.R, Shukla.A. Cytogenetic studies in Down Syndrome. Indian Pediatr.1991; 9: 991-6.
- Tamim H. Shaikh, Marcia L. Budarf, Livija Celle, Elaine H. Zackai and Beverly S. Emanuel. Clustered 11q23 and 22q11 Breakpoints and 3:1 Meiotic Malsegregation in Multiple Unrelated t(11;22) Families. J. Hum. Genet. 1999; 65:1595–1607.
- Sayee Rajangam, Ph.D., Preetha Tilak, M.B.B.S., Aruna N, M.S., D.N.B., Rema Devi, M.S., D.N.B. Karyotyping and counseling in bad obstetric history and infertility. Iranian Journal of Reproductive Medicine., Winter 2007; 15: 7-12.
- Dong Y,Du RC, Jiang YT, Wu J, Li LL, Liu RZ. Impact of chromosomal translocations on male infertility, semen quality, testicular volume and reproductive hormone levels. J Int Med Res., 2012; 40(6):2274-83.
- J. Vijayalakshmi, P. Venkatchalam, Solomon F.D. Paul, G. Usha Rani, P. Kumarasamy and Jayam Kannan. Chromosomal Anomalies in Patients with Azoospermia and Oligoasthenoteratozoospermia. Int J Hum Genet., 2011; 11(2): 117-121








