Syed Imam Rabbani¹, Kshama Devi¹* and Salma Khanam
1Department of Pharmacology,Al-Ameen College of Pharmacy, Opp. Lalbagh, Main Gate, Hosur Road, Bangalore- 560 027 India.
2Department of Pharmacognosy, Al-Ameen College of Pharmacy, Opp. Lalbagh, Main Gate, Hosur Road, Bangalore- 560 027 India.
Corresponding Author E-mail: devikshama@gmail.com
Abstract
Oxidative stress in type-2 diabetes mellitus (T2DM) is the major cause for the infertility in male diabetic patients. In this study, the role of Metformin (Met-5, 50 and 500 mg/kg, po for 4 weeks) was evaluated against the reproductive damage induced by nicotinamide (230 mg/kg, ip) and streptozotocin (STZ-65 mg/kg, ip) in experimental T2DM male Wistar rats. The reproductive toxicity was evaluated by determining the caudal sperm shape abnormality, sperm count and weight of the testis. Further, serum lipid peroxidation (LPO), glutathione (GSH), glutathione peroxidase (GPx) and blood glucose levels were tested to find the relationship between oxidative stress, hyperglycemia and injury to the male reproductive cells. Administration of Met at 500 mg/kg significantly (P<0.01) minimized the sperm abnormalities and increased (P<0.05) the weight of the testis compared to the diabetic rats. Met (500 mg/kg) treatment also reduced the LPO level (P<0.05) and increased (P<0.01) the GSH and GPx levels besides exhibiting anti-hyperglycemic effect (p<0.001) compared to the diabetic animals. Met at 50 mg/kg prevented (P<0.05) the sperm shape abnormality and increased the (P<0.05) the GSH level along with a anti-diabetic effect (P<0.001), however, the lower dose of Met (5 mg/kg) did not alter the NA-STZ mediated changes in the diabetic state. The results indicated that the antioxidant property of Met could be responsible for preventing the hyperglycemia mediated sperm abnormalities in the diabetic condition.
Keywords
Metformin; anti-diabetic; sperm shape abnormality; sperm count; antioxidant
Download this article as:| Copy the following to cite this article: Rabbani S. I, Devi K, Khanam S. Effect of Metformin Against the Nicotinamide-Streptozotocin Induced Sperm Abnormalities in Diabetic Male Wistar Rats. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: Rabbani S. I, Devi K, Khanam S. Effect of Metformin Against the Nicotinamide-Streptozotocin Induced Sperm Abnormalities in Diabetic Male Wistar Rats. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=594 |
Introduction
Oxidative stress due to hyperglycemia in diabetes mellitus type-2 (T2DM) is known to play major role in the fertility related health complications in diabetic patients. Human sperm cells are reported to be highly sensitive for the oxidative damages. As a consequence of the oxidative injury, spermatozoa undergo peroxidation leading to DNA fragmentation in the nuclear components of the cells1. The reactive oxygen species (ROS) induced sperm injury results in both qualitative and quantitative loss and believed to be responsible for male infertility. At lower levels of oxidative damage, spermtazoa might retain the capacity for fertilization while still carrying significant amount of mutation. The subsequent repair of such mutation in the zygote is reported to contribute in complications including childhood carcinogenesis, neurological defects etc2.
Metformin (Met) HCl is a biguanide derivative producing an antihyperglycemic effect mainly in diabetic condition. Met reported to have no effects on the pancreatic beta cells3. The mode of action has been postulated that Met might enhance the effect of insulin on the peripheral receptor site as well as increase in the number of insulin receptors on the cell surface membranes4. Met in the clinical studies is reported to be useful in the treatment of polycystic ovary syndrome, obesity, hypertension, neoplasia etc5-8. Met also reported to have favorable effects against dyslipidemia, reduction in pro-inflammatory cytokines and monocyte adhesion molecules and improved glycations status, thus benefiting endothelial function in the macro- and micro- vasculature9. In the earlier study, Met was reported to modulate the action of ROS generated by both invivo and invitro models10,11. In addition, Met has been reported to possess anti-clastogenic activity against the cytotoxic effects of adriamycin in swiss albino mice and this activity was related to the antioxidant potential. Earlier research suggests that drugs having the ability to reduce the oxidative stress related nuclear damage can also be useful in overcoming the ROS mediated reproductive complications including infertility1,2, hence we designed the present study to evaluate the role of Met against the nicotinamide-streptozotocin induced sperm abnormalities in diabetic male Wistar rats.
Materials and methods
Chemicals
A gift sample of Metformin (Met) was obtained from Micro Lab Ltd, Bangalore. The stains and other reagents/chemicals used in this study were of analytical grade and procured from the regular suppliers.
Animals
Eight week-old healthy, laboratory bred, male Wistar rats weighing 180 ± 10 gm were maintained under standard laboratory conditions such as temperature 20±2o C, 12 hour light / dark cycle and provided water and pellet food ad libitum. The experiments were conducted in CPCSEA (Committee for the purpose of control and supervision of experiments on animals, Chennai, India) approved animal house after obtaining the prior approval from the Institutional Animal Ethics Committee (AACP/IAEC/P-31/2005).
Induction of Type-2 diabetes
Experimental T2DM was developed in adult rats by administering streptozotocin (STZ) and nicotinamide (NA)13. The animals received intraperitoneal administration of NA – 230 mg/kg (SD Fine-Chem Ltd, Mumbai, India) dissolved in saline 15 min before an administration of STZ – 65 mg/kg, ip (Sigma Aldrich, USA) dissolved in 0.1 M citrated buffer (pH 4.5) immediately before use. Blood glucose was estimated after 2 days and the animals with glucose level ≈ 180 ± 8 mg/dl are only selected for the study.
Dosage, treatment and sampling
The animals were divided mainly in to three groups ie., control, diabetic and treatment, consisting of eight animals in each group. The treatment group received three doses of Met viz., 5, 50 and 500 mg/kg 14-16 orally per day as gavage for 4 weeks after the induction of diabetes. The control and diabetic animals were administered saline (0.5 ml/kg) daily through out the treatment period. In this study, α-tocopherol (20 mg/kg, po, 4 weeks)17 and insulin (3 IU/kg, sc, 4 weeks)18 were used as standard antioxidant and hypoglycemic agents, respectively. Before the administration, α-tocopherol was suspended in 1% w/v carboxy methyl cellulose (CMC) whereas Met was dissolved in distilled water and insulin was reconstituted in water for injection to obtain the required dose.
Sperm morphology and sperm count assay:
The procedure described by Wyrobek and Bruce (1975)19 was followed to study the sperm shape abnormality in cauda epididymis of the rats. One thousand sperms per animals were screened to find the different types of abnormality in one of the cauda epididymis. Five types of abnormalities such as amorphous, hookless, banana shape, fused and double headed (Photo-1) were evaluated and finally represented as percentage total abnormality20.
The caudal sperm count test was performed according to D’Souza (2004). The spermatozoa count was obtained by counting the number of sperm cells in the four WBC chambers using a neubauer’s slide21.
In vivo antioxidant activity
Blood samples were collected from the retro-orbital plexus under light ether anesthesia. The serum was separated by centrifugation (1000 rpm) and immediately analyzed to determine the antioxidant enzyme activity.
Serum lipid peroxidation (LPO)
The procedure described by Ohkawa et al (1979) was followed to estimate the lipid peroxidation. The principle depends on the reaction between thiobarbituric acid with malondialdehyde, a secondary product of lipid peroxidation at pH 4. A reddish pink color developed was estimated at 532 nm which indicates the extent of peroxidation. The extent of lipid peroxidation was expressed as η mol/ mg protein22.
Glutatione reduced (GSH)
The reduced glutathione (GSH) was estimated using the procedure described by Beutler et al (1963). The sulfhydril groups present in the glutathione forms a colored complex with DTNB {5, 5’ dithiobis – (2-nitrobenzoic acid)}, which was measured colorimetrically at 412 nm. GSH levels were expressed as μg / mg protein 23.
Glutathione peroxidase (GPx)
GPx (EC 1.11.1.9) activity was assayed based on the modified method of Paglia and Valentine described by Heath and Tappel (1976). A 100 μl of the serum sample is incubated for 5 min at 37o with stock solution (0.25 mM GSH, 0.12 mM NADPH and 1 unit of glutathione reductase prepared in the tris buffer) in a final volume of 1.65 ml. 50 μl of cumene hydroperoxide (1 mg/ml) are added to start the reaction, and the absorbance at 340 nm is monitored for the rate of disappearance of NADPH and the GPx value was represented as μg of glutathione consumed/min/mg protein24.
Blood glucose estimation
A drop of blood was collected from the tail vein and applied to the test zone of the glucose strip for immediate measurement of the fasting glycemia (mg / dl) using the Ascensia ENTRUST glucometer (Bayer healthcare Ltd, Mumbai).
Statistics
The statistical analyses of the result for the sperm abnormality was done by One-way Anova followed by Mann- Whitney U test25 and the antioxidant data was analyzed by student ‘t’ test followed by One way Anova. P<0.05 was considered to indicate the significance.
Results
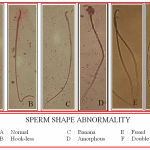 |
Figure 1:
|
Effect of Metformin on the sperm abnormality in NA-STZ induced diabetic rats.
The experimental diabetic condition after the administration of NA and STZ produced significant (P<0.001) increase in the sperm shape abnormality and reduced the caudal sperm count along with decreased weight of the testis when compared with the normal animals. Met at 500 mg/kg significantly (P<0.01) suppressed the sperm shape abnormality and enhanced the sperm count and also increased the weight of testis compared to the diabetic animals. However, the lower doses of Met (5 and 50 mg/kg) did not alter the NA-STZ mediated changes in sperm shape abnormality, sperm count and weight of testis, although the median tested dose of Met (50 mg/kg) inhibited (P<0.05) the sperm morphological changes compared to the diabetic group. α-tocopherol (20 mg/kg) exhibited a significant (P<0.01) suppression in the sperm abnormalities but did not increase the weight of testis in diabetic rats. Further, administration of insulin (3 IU/kg) did not alter the sperm shape abnormality, sperm count and weight of testis in diabetic condition (Table-1).
Table 1: Effect of Metformin on the Sperm morphology and sperm count in NA-STZ induced diabetic rats.
|
Sperm abnormality test
|
Treatment and Dose
(mg/kg) |
||||||
|
Control (Saline- 0.5ml / kg)
|
NA (230 mg) + STZ (65 mg) |
NA-STZ + Metformin (5 mg/kg)
|
NA-STZ + Metformin (50 mg/kg) |
NA-STZ + Metformin (500 mg/kg)
|
NA-STZ + α-Tocopherol (20 mg/kg)
|
NA-STZ + Insulin (3 IU/kg) |
|
|
Weight of Testis (gm)
|
1.23 ± 0.02 | 1.18 ± 0.04 a | 1.19 ± 0.03 a | 1.22 ± 0.03 | 1.26 ± 0.05 * | 1.19 ± 0.07a | 1.19 ± 0.02 a |
|
Total % Abnormality
|
1.04 ± 0.07 | 1.64 ± 0.07 c | 1.66 ± 0.08 c | 1.50 ± 0.11 c * | 1.42 ± 0.09 c ** | 1.21 ± 0.34 ** | 1.52 ± 0.18 c |
|
Sperm count (106)
|
33.18 ± 1.36 | 27.77 ± 1.31 c | 27.90 ± 2.19 c | 29.03 ± 3.17 c | 31.05 ± 1.19 b ** | 31.87 ± 2.76 ** | 27.44 ± 2.63 c |
Values are expressed as Mean ± SD, NA – Nicotinamide, STZ – Streptozotocin, N=8
Statistics: One way Anova followed by Mann-Whitney U test,
a p<0.05, b p<0.01, c p<0.001 compared with the control
*p<0.05, **p<0.01 compared with the Diabetic group
Effect of Metformin on serum antioxidant status and glucose level in NA-STZ induced diabetic rats.
The administration of NA-STZ had produced significant (P<0.001) increase in lipid peroxidation (LPO) and blood glucose level and reduced the serum levels of GSH and GPx compared to the control animals. Three doses of Met (5, 50 and 500 mg/kg) tested in the diabetic animals indicated that Met at 500 mg/kg significantly enhanced the serum levels of GSH (P<0.01), GPx (P<0.05) besides reducing the LPO (P<0.05) and blood glucose levels (P<0.001) compared to the diabetic rats. Met at 50 mg/kg exhibited significant (P<0.001) anti-hyperglycemic effect but did not alter the serum levels of LPO, GPx, although the GSH level was observed to be elevated (P<0.05) compared to the NA-STZ diabetic condition. However, the lower dose of Met (5 mg/kg) did not cause any change in the antioxidant status and blood glucose level in T2DM. Further, administration of α-tocopherol improved (P<0.01) the antioxidant status and also reduced (P<0.05) the hyperglycemic condition. The treatment with insulin showed potent (P<0.001) anti-hyperglycemic effect in the diabetic condition but the oxidative stress was found to be unaltered compared to the NA-STZ treated animals (Table-2).
Table 2: Effect of Metformin on the serum antioxidant status and glucose level in NA-STZ induced diabetic rats.
|
Serum antioxidant status and glucose level |
Treatment and Dose
(mg/kg) |
||||||
|
Control (Saline- 0.5ml / kg)
|
NA (230 mg) + STZ (65 mg) |
NA-STZ + Metformin (5 mg/kg)
|
NA-STZ + Metformin (50 mg/kg) |
NA-STZ + Metformin (500 mg/kg)
|
NA-STZ + α-Tocopherol (20 mg/kg)
|
NA-STZ + Insulin (3 IU/kg) |
|
|
Lipid peroxidation ( η mol/ mg protein ) |
2.39 ± 0.20 | 3.35 ± 0.22 c | 3.37 ± 0.28 c | 3.24 ± 0.54 c | 3.11 ± 0.04 c * | 2.40 ± 0.37*** | 3.31 ± 0.36 c |
|
Glutathione (GSH) (μg / mg protein) |
3.27 ± 0.12 | 1.748 ± 0.27 c | 1.76 ± 0.30 c | 2.09 ± 0.21 c * | 2.26 ± 0.27 c ** | 2.84 ± 0.52 ** | 1.75 ± 0.14 c |
|
Glutathione Peroxidase (GPx) (μg of glutathione consumed / mg protein) |
1.52 ± 0.04 | 1.19 ± 0.17 c | 1.18 ± 0.25 c | 1.27 ± 0.17 b | 1.48 ± 0.27 a * | 1.41 ± 0.10 a * | 1.17 ± 0.29 c |
|
Blood glucose (mg/dl) |
92.3 ± 3.44 | 174.3 ± 6.32 c | 168.47 ± 7.79 c | 140.9 ± 7.62 c *** | 128.8 ± 6.66 c *** | 157.4 ± 6.47 c * | 120.4 ± 6.47 c *** |
Values are expressed as Mean ± SD, NA – Nicotinamide, STZ – Streptozotocin, N=8
Statistics: Student ‘t’ test followed by One way Anova..
ap<0.05, bp<0.01, cp<0.001 compared with the Control
*p<0.05, **p<0.01, ***p<0.001 compared with the Diabetic group
Discussion
Present research indicated that administration of NA-STZ produced moderate hyperglycemia (blood glucose ≈ 180 ± 8 mg/dl). The diabetic condition increased the sperm shape abnormality, reduced sperm count and weight of testis besides increasing the oxidative stress and blood glucose level (Table-1 and 2). The partial protective effect of NA against the STZ induced β-cytotoxicity is reported to mimic the clinical T2DM. Further, the moderate and sustained hyperglycemia due to the co-administration of NA and STZ was observed to increase the life span of the diabetic animals hence this diabetic model in rats is suitable for the long term studies. NA protects the STZ-mediated cellular injury by preserving the intracellular NAD pool either by acting as a precursor of NAD or by inhibiting the activity of poly (ADP-ribose) synthetase which is a NAD consuming enzyme activated by the STZ13.
Earlier study indicated that hyperglycemia in T2DM contributes in the generation of ROS thus increasing the oxidative stress in the diabetics. Although, testis is protected with several antioxidant enzymes like GSH, SOD, catalase, GPx etc, their concentrations are considerably lower to prevent the damage to the spermatozoa26. ROS generated in the hyperglycemic condition after the administration of STZ is reported to cause sperm abnormalities and the mechanism suggested include the activation of formation of advanced glycation end products (AGE), polyol pathway, hexosamine pathway and protein kinase C (PKC)27.
Sperm shape abnormality and sperm count assays are routinely employed to determine the role of disease/drugs on the male germinal cells. Test to determine the variation in the sperm morphology has special emphasis as these assays help to identify the defective sperms which can act as the carriers for the genetic abnormalities in the progeny25.
The present study indicated that administration of α-tocopherol (20 mg/kg) and Met (500 mg/kg) prevented the NA-STZ intervened sperm irregularities and enhanced the antioxidant status in the diabetic animals along with reducing the blood sugar levels (Table1 and 2). The anti-hyperglycemic activity of α-tocopherol has been linked to its antioxidant potential28. α-tocopherol in this study reduced the serum LPO and enhanced the GSH and GPx levels in the diabetic animals. LPO occurs when ROS attacks the polyunsaturated fatty acid residues of phospholipids of the cell membrane. Spermatozoa are highly sensitive to ROS due to high content of polyunsaturated fatty acid in its plasma membrane29. The function of GPx is to remove the H2O2 generated by metabolic action or oxidative stress. The activity of GPx is highly dependent on GSH concentration. GSH scavenges peroxynitrite and HO. as well as convert H2O2 to water with the help of GPx29,30. The ability of α-tocopherol to reverse the changes in the serum levels of LPO, GSH and GPx and sperm defects indicated that compounds containing the antioxidant activity could prevent the reproductive damages caused by ROS26. However, the inability of α-tocopherol to enhance the weight of testis in diabetic animals show that the tested dose and duration of α-tocopherol did not averted the NA-STZ mediated tubular damage and also did not augment the proliferation of seminiferous, interstitial and other cells25. Further, administration of insulin (3 IU/kg) although exhibited potent antidiabetic effect but failed to prevent the reproductive abnormalities and oxidative stress in diabetic rats. These findings suggest that compounds possessing hypoglycemic activity alone in the diabetic patients might not prevent the oxidative damages on the reproductive organs.
Treatment with Met (500 mg.kg) prevented the NA-STZ induced defects in sperm shape, sperm count, weight of testis and oxidative stress besides exhibited antidiabetic activity (Table-1 and 2). Interestingly, Met at 500 mg/kg enhanced the weight of testis in the diabetic animals. The data suggest that Met might have stimulated the proliferation of cells of testicles in the diabetic animals. However, the lower doses of Met (5 and 50 mg/kg) could not elicit similar response indicating lack of intrinsic activity at lower dose in diabetic condition. The antioxidant mechanism of Met is already reported in the literature. The mechanism includes inhibition of NADPH-oxidase, the enzyme responsible for the production of ROS mainly by activation via phospholipase C and an increase in Ca2+ influx10,12. Similar mechanism involving inhibitory action of NADPH oxidase enzymes by Met has been reported to suppress the formation of ROS in bovine aortic endothelial cells31. Considering these information it can be suggested that Met exhibited similar mechanism responsible for decreasing the ROS levels and might have also stimulated the generation of antioxidant enzymes or preserved them form getting exhausted during the oxidative stress.
To conclude, the present study indicated that compounds owning anti-diabetic and antioxidant activities like Met could benefit the diabetic patient in reducing the oxidative stress related reproductive complications.
References
- Rehman K., Beshay E. and Carrier S. Reprod. Med., 1: 29 (2001).
- Olsen A.K., Lindeman B., Wige R., Duale N. and Brunborg G. Toxicol. Appl. Pharmacol., 207: S521 (2005).
- Krentz A.J and Bailey C.J. Drugs. 65: 384 (2005).
- Wiernsperger N.F. and Bailey C.J. Drug. 58: 31 (1999).
- Meenakumari K.J., Agarwal S., Krishna A. and Pandey L.K. Brazilian J. Med. Biol. Res., 37: 1637 (2004).
- Baptista T., Raugel W., Fernandez V., Carrizo E. and Fakih Y.E. Schizophrenia Res., 93: 99 (2007).
- Bhalla R.C., Toth K.F., Tan E., Bhatty R.A. and Mathias E. Am. J. Hyperten., 9: 570 (1996).
- Gotlieb W.H., Saumet J., Beauchamp M.C., Gu J. and Lau S. Gynecologic Oncol., 110; 246 (2008).
- Bailey C.J. Cardiovasc. Drugs Ther., 8: 92 (2008).
- Tanaka Y., Iwamoto H., Onuma T. and Kawamori R. Curr. Ther. Res., 58: 693 (1997).
- Liu Z., Li J., Zeng Z., Liu M. and Wang M. Chemico-Biological Interactions., 173: 68 (2008).
- Aleisa A.M., Al-Rejaie S.S., Bakheet S.S., Al-Bekari A.M. and Al-Shabanah O.A. Mutat. Res., 634: 93 (2007).
- Masiello P., Broca C., Gross R., Roye M., Manteghetti M. and Hillaire-Buys D. Diabetes. 47: 224 (1998).
- Jin H.E., Hong S.S., Choi M.K., Maeng H.J., Kim D.D., Chung S.J. and Shim C.K. Pharmacol. Res., 26: 549 (2008).
- Vilmaz B., Aksakal O., Gungor T., Sirvan L., Sut N., Kelekci S., Soyyal S and Mollamohmutoglu L. Reprod. Biomed. Online., 18: 436 (2009).
- Waisundara V.Y., Huang M., Hsu A., Huang D. and Tan B.K. Am. J. Chin. Med., 36: 1083 (2008).
- Ihara Y., Yamada Y., Toyokuni S., Miyawaki K., Ban N. and Adachi T. FEBS. Lett. 473: 24 (2000).
- Destefano M.B., Stern J.S. and Castonguary T.W. Physiol. Behav., 50: 801 (1991).
- Wyrobek A.J. and Bruce W.R. Proc. Natl. Acad. Sci., U S A, 72: 4425 (1975).
- Narayana K., D’Souza U.J.A. and Rao K.P.S. Mutat Res., 513: 193 (2002).
- D’Souza U.J.A. Asian J. Androl., 6: 223 (2004).
- Ohkawa H., Ohishi N. and Yagi K. Anal. Biochem., 95: 351 (1979).
- Beutler E., Duron O. and Kelly B. M. J. Lab. Clin. Med., 61: 882 (1963).
- Heath R.L. and Tappel A.L. Anal. Biochem., 76: 184 (1976).
- Rai S.P. and Vijayalaxmi K.K. Mutat. Res., 492: 1 (2001).
- Tramer F., Rocco F., Micali F., Sandri G. and Panfili E. Biol. Reprod., 59: 753 (1998).
- Shrilatha B. and Muralidhara. Reprod. Toxicol., 23: 578 (2007).
- Krishnamoorthy G., Venkataraman P., Arunkumar A., Vignesh R.C., Aruldas N.M. and Arunakaran J. Reprod. Toxicol., 23: 239 (2007).
- Piconi L., Quagliar O. and Ceriello. Clin. Chem. Lab. Med., 41: 1144 (2003).
- Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M. and Telser J. Int. J. Biochem. Cell. Biol., 39: 44 (2007).
- Quslimani N., Peynet J., Bonnefont-Rousselot D., Therond P., Legrand A. and Beaudeux J.L. Metab. Clin. Exp., 54: 829 (2005).







