Nadeem Siddiqui¹*, M. Shamsher Alam¹, Sharique Ahmed², Ruhi Ali¹, M. Faiz Arshad¹ and Waquar Ahsan¹
¹Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Hamdard University, Hamdard Nagar, New Delhi - 110 062 India.
²Department Biochemistry, Faculty of Medicine, 7th October University, Misurata India.
Corresponding Author E-mail:nadeems_03@rediffmail.com
Abstract
Sepracor Inc. (Nasdaq: SEPR) announced the presentation of Phase III combined clinical results for eslicarbazepine acetate (SEP-0002093 / BIA 2-093) in the treatment of epilepsy at the Ninth Eilat Conference on New Anti-Epileptic Drugs in Spain. The study demonstrates that eslicarbazepine is well tolerated and effective when used as adjunct therapy for adult patients with partial epilepsy with distinctively lesser side effects. The pharmacological and computational properties of eslicarbazepine have been studied and discussed.
Keywords
Eslicarbazepine; Pharmacokinetics; Antiepileptic; Computational
Download this article as:| Copy the following to cite this article: Siddiqui N, Alam M. S, Ahmed S, Ali R, Arshad M. F, Ahsan W. Pharmacological and Computational Approach to Eslicarbazepine: A Short Review. Biomed. Pharmacol. J.;2(1) |
| Copy the following to cite this URL: Siddiqui N, Alam M. S, Ahmed S, Ali R, Arshad M. F, Ahsan W. Pharmacological and Computational Approach to Eslicarbazepine: A Short Review. Biomed. Pharmacol. J.2009;2(1)). Available from: http://biomedpharmajournal.org/?p=546 |
Introduction
Epilepsy is one of the most common neurological diseases and, according to the Epilepsy Foundation; approximately 2.7 million people in the U.S. have epilepsy. Epilepsy is characterized by abnormal firing of impulses from nerve cells in the brain. Treatment of partial seizures, the most common type of epilepsy, presents a constant challenge – up to 58 percent of patients with partial seizures do not achieve seizure control with current anti-epileptic drugs. The classical antiepileptic drugs comprise phenobarbital, available since 1911; phenytoin, marketed in 1939; carbamazepine, used for epilepsy in Europe from the mid 1960s; and valproic acid,1 available in several European countries as of the late 1960s. All currently approved antiepileptic drugs have dose-related toxicity and idiosyncratic side effects.2 Eslicarbazepine (ESL) is a novel, voltage-gated sodium channel blocker that has been studied to reduce the frequency of partial-onset seizures when used in combination with other anti-epileptic drugs. ESL shares with carbamazepine and oxcarbazepine the dibenzazepine nucleus bearing the 5-carboxamide substitute, but is structurally different at the 10, 11-position. This molecular variation results in differences in metabolism, preventing the formation of toxic epoxide metabolites such as carbamazepine-10, 11-epoxide.
Pharmacology
Mechanism of action
Mechanistically, ESL does not interfere with receptors for benzodiazepines, gamma-aminobutyric acid (GABA), and glutamate. It behaves as a potent blocker of voltage-gated sodium channel (VGSC) by competitively interacting with site 2 of the inactivated state of the channel.3
Pharmacokinetics
Eslicarbazepine acetate is rapidly and extensively metabolized by first-pass metabolism to its main active metabolite, eslicarbazepine (S-licarbazepine). There were more subjects with measurable plasma concentrations of the parent drug (ESL) in the hepatic impairment group than in the control group, suggesting that first-pass metabolism was slightly decreased by liver impairment. However, ESL plasma concentrations remained very low, representing only about 0.01% of total systemic exposure.4 The pharmacokinetics of ESL was not affected by moderate hepatic impairment. Therefore, patients with mild to moderate liver impairment treated with ESL do not require dosage adjustment.
Indications and Usage
The active substance of Zebinix is Eslicarbazepine acetate, an antiepileptic medicinal product (N03AF04) that is a prodrug of eslicarbazepine.5 On 19 February 2009 the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending granting a marketing authorization for the medicinal product Zebinix, 400 mg, 600 mg, 800 mg, tablets intended for adjunctive therapy in adults with partial-onset seizures with or without secondary generalization.
Adverse effects
The most common side effects are dizziness, somnolence, headache, abnormal coordination, disturbed vision, nausea, rash, and fatigue.5
Computational Analysis
IUPAC name: 10-methyl-10,11-dihydro-5H-dibenzo[b,f]azepine-5-carboxamide
Molecular formula: C16H16N2O, Molecular Weight: 252.3
Physicochemical properties
The physicochemical properties of Eslicarbazepine were calculated from the software ChemBioDraw Ultra 11.0 version and ACD Labs 2.0 version and the values are presented in Table 1.
Spectral analysis
The 1H-NMR and the 13C-NMR were analyzed by using the software ChemBioDraw Ultra11.0 version and the chemical shift values are presented as below.
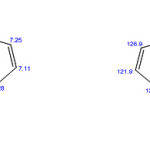 |
Scheme 1:
|
Pharmacophore modeling
Dimmock et al6, 7 proposed the interaction at the binding site. The pharmacophoric elements essential for good anticonvulsant activity were thought to be a lipophilic aryl ring and hydrogen-bonding moiety. It is imaginable from the structure of Eslicarbazepine (Fig. 1) that the structural features essential to interact at the binding site were a lipophilic aryl ring A, hydrogen acceptor HA, and a hydrogen donor HD.
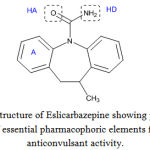 |
Figure 1: Structure of Eslicarbazepine showing presence of essential pharmacophoric elements for anticonvulsant activity.
|
Table 1: Physicochemical parameters of Eslicarbazepine.
| S. No. | Properties | Value |
| 1. | Log Pa | 3.3 |
| 2. | Molar refraction | 74.78 ± 0.3 cm3/mol |
| 3. | Clog Pb | 2.65 |
| 4. | CMRc | 7.65 |
| 5. | Molar volume | 213.2 ± 3.0 cm3 |
| 6. | Refractive index | 1.61 ± 0.02 |
| 7. | Surface tension | 48.8 ± 3.0 dyne/cm |
| 8. | Density | 1.18 ± 0.06 g/cm3 |
| 9. | Polarizability | 29.64 ± 0.5 10-24cm3 |
| 10. | Parachor | 563.8 ± 6.0 cm3 |
aPartitioning coefficient; bCalculated partitioning coefficient;
cCalculated molar refraction.
Three-Dimensional structure analysis
The three dimensional structure of Eslicarbazepine was studied by using the software Ortep3v2. The ortep diagram of the drug was drawn at 50% probability and it is shown in Fig. 2.
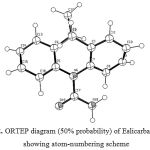 |
Figure 2: ORTEP diagram (50% probability) of Eslicarbazepine showing atom-numbering scheme.
|
Crystal data
Crystal data were calculated from the software and presented below
Unit cell length (Å): a = 10.00, b = 10.00, c = 10.00
Unit cell angles (deg): α = 90.00, β = 90.00, γ = 90.00
Atomic species C N O H
No. in asymm. unit: 16.00 2.00 1.00 16.00
No. in unit cell : 16.00 2.00 1.00 16.00
Covalent radius (Å) 0.77 0.74 0.66 0.37
Crystal class: Triclinic
Conclusion
Eslicarbazepine is regarded as a good candidate in the drug discovery of epilepsy showing high efficacy with minimal side effects. The computational parameters of the drug were determined to study the silico conformational behavior and the physicochemical properties for further QSAR analysis.
References
- Hess, R., Frey, H. H., Janz, D. “In Antiepileptic Drugs’’ Springer-Verlag: Berlin; 35 (1985).
- Brodie, M. J. “Established anticonvulsants and treatment of refractory epilepsy’’. Lancet. 336-350 (1990).
- Benes, J., Parada, A., Figueiredo, A. A., Alves, P. C., Freitas, A. P., Learmonth, A., Cunha, R. A., Garrett, J., Soares-da-Silva, P. “Anticonvulsant and sodium channel-blocking properties of novel 10, 11-dihydro-5H-dibenz [b, f] azepine-5-carboxamide derivatives.’’ J. Med. Chem. 42: 2582–2587 (1999).
- Almeida, L., Potgieter, J. H., Maia, J., Potgieter, M. A., Mota, F., Soares-da-Silva, P. “Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment’’. Eur J. Clin. Pharmacol. Mar; 64:267-273 (2008).
- European Medicines Agency Pre-Authorization Evaluation of Medicines for Human Use. London, 19 February 2009.
- Dimmock, J. L and Puthucode, R. N. “(Aryloxy)aryl Semicarbazones and Related Compounds: A Novel Class of Anticonvulsant Agents Possessing High Activity in the Maximal Electroshock Screen.” Med. Chem. 39:3984-3997 (1996).
- Puthucode, R. N., Pugazhenthi, U., Quail, J. W. “Anticonvulsant activity of various aryl, arylidene and aryloxyaryl semicarbazones.” J. Med. Chem. 33:595-607 (1998).







