Manuscript accepted on :
Published online on: --
Plagiarism Check: Yes
M. M. Mohamadein1, R. M. Farrag2. and. A. A. I. Mekawey2
1The Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo-Egypt 2Collage of Applied Medical Science, Prince Sattam Bin AbdulAziz University, Al-Kharj, KSA.
DOI : https://dx.doi.org/10.13005/bpj/800
Abstract
In vitro activity of EHP [1-(2-Ethyl, 6-Heptyl) Phenol], a natural compound purified from cumin seeds extract, was investigated against ten dermatophytic isolates; Trichophyton (n=5), Microsporum (n=4), and Epidermophyton (n=1), as well as three viruses; HAV, Cox B4, and HSV-1. The highest antidermatophtic activity was against T. fulvum and E. floccosum while T. terrestre exhibited the most resistant isolate followed by T. schoenleinii. MIC of EHP ranged from 2.5 to 25 µg/ml, however owing to cytotoxicity results, EHP concentrations up to 10 µg/ml are preferably used. EHP possessed antiviral activity against the investigated viruses with HAV being the most affected followed by Cox B4 and then HSV1 with a plaque reduction percentage of 70, 65, and 60, respectively.
Keywords
Cuminum cyminum; Viruses; Dermatophytes; EHP
Download this article as:| Copy the following to cite this article: Mohamadein M. M, Farrag R. M, Mekawey A. A. I. Antiviral and Antidermatophytic Activity of a Compound Extracted from Cuminum Cyminum Seeds. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Mohamadein M. M, Farrag R. M, Mekawey A. A. I. Antiviral and Antidermatophytic Activity of a Compound Extracted from Cuminum Cyminum Seeds. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=4891 |
Introduction
Plants are safe to human and the ecosystem, and can easily be used by the public who used them for thousands of years to enhance flavor and aroma of foods as well as its economic value (Aly et al., 2000). These natural plants involve garlic, lemon grass, cumin, datura, acacia, a triplex, ginger, black seed, neem, basil, eucalyptus, alfalfa and basil (Aly and Bafiel, 2008).
Additionally, spices and herbs have been used traditionally for thousands of years by many cultures not only as flavouring agents but also as food preservatives. They are generally recognised as safe because of their traditional use without any documented detrimental impact. They are also inexpensive, show better patient tolerance and are readily available for low socioeconomic population (Bag, A. and Chattopadhyay, R.R., 2015)
A popular spice in world, cumin is rich in iron, an important mineral for immune health. Cumin has also been used traditionally to improve digestion, and preliminary scientific evidence suggests that its traditional reputation may be justified. Animal studies indicate that cumin may have anti-carcinogenic properties as a result of its antioxidant content and ability to enhance detoxification enzymes in the liver, protecting against the formation of liver and stomach tumors.Cumin seeds were also reported to possess antimicrobial activities for different microorganisms, including bacterial strains, yeasts and fungi (De et al., 2003).
Dermatophytes are fungi that can cause infections of the skin, hair, and nails due to their ability to utilize keratin. The organisms colonize the keratin tissues and inflammation is caused by host response to metabolic by-products. The dermatophytes are included in three fungal genera viz,:1. Epidermophyton: This genus consists of 2 species, one of which is a pathogen 2. Microsporum: There are 19 described species but only 9 are involved in human or animal infections. 3. Trichophyton: There are 22 species, most causing infections in humans or animals (Indira,G. 2014)
In the past few decades, a worldwide increase in the incidence of fungal infections has been observed as well as a rise in the resistance of some fungal species to different fungicidals used in medicinal practice. Fungi are one of the most neglected pathogens, as demonstrated by the fact that the amphotericin B, a polyene antibiotic discovered 1956, is still used as a “gold standard” for antifungal therapy. The last two decades have witnessed a dramatic rise in the incidence of life threatening systemic fungal infections (Abad et al., 2007).
Unlike the search for antibiotics, which took root from the discovery of penicillin late 1930s, the search for antiviral agents began in the 1950s but had a breakthrough in 1964. Early success in this direction included the use of methisazone for the prophylaxis of small pox and the use of idoxuridine for the treatment of herpes keratitis (Kinchington et al., 1995).
Two major obstacles to the development and use of effective antiviral chemotherapy are the close relationship that exists between the multiplying virus and the host cell, and that viral diseases can only be diagnosed and recognized after it is too late for effective treatment. In the first case, an effective antiviral agent must prevent completion of the viral growth cycle in the infected cell without being toxic to the surrounding normal cells (Desselberger, 1995). One encouraging development is the discovery that some virus specific enzymes are elaborated during multiplication of the virus particles and this may be a point of attack by a specific inhibitor. However, recognition of the disease state too late for effective treatment would render that antiviral agent useless even if they were available.
Until early recognition of the disease state is provided, most antiviral chemotherapeutic agents will have their value as prophylactic agents. The reason for the apparent lack of progress in antiviral chemotherapy as compared with the field of antibacterials has been a problem of selectivity (Kinchington, 1995). Any antiviral agent must selectively kill the pathogenic organism in the presence of other living cells. Sufficient biochemical differences exist between the metabolism of prokaryotic bacterial cells and mammalian cells to enable selectivity to be achieved, hence the early development of antibacterial agents, which were safe for human use. Viruses on the other hand, despite their apparent simplicity present a bigger problem. This is because during their replicative cycle, they become physically and functionally incorporated into the host cells and it is therefore difficult to distinguish unique biochemical features suitable for selective attack.
Some viruses also persist in a latent infection (Cann, 1993), in which case, antiviral drugs are less likely to be effective. However increased understanding of the molecular events of virus infections has meant that the search for antiviral drugs against specific targets can be conducted on a more rational basis(Abonyi et al., 2009).
Early cultures also recognized the value of plant materials in medicine. Plant extract has been used traditionally to treat a number of infectious diseases including those caused by bacteria, fungi, protozoa and viruses (Soylu et al., 2005). In the recent years, researches on medicinal plants have attracted a lot of attention globally. Large body of evidence has accumulated to demonstrate the promising potential of medicinal plants used in various traditional, complementary and alternate systems of treatment of human diseases. Plants are rich in a wide variety of secondary metabolites such as tannins, terpenoids, alkaloids, flavonoids, etc, which have been found in vitro to have antimicrobial properties (Yoshida et al., 2005).
Medicinal plant products have been used as folk remedies for different kinds of ailments including viral diseases(Field and Biron, 1994). There is a need to search for new compounds for treatment of viralinfections since there is an increasing resistance to antiviral drugs .Traditional plant extracts having anti-infective properties, have been screened for their antiviral activity (Chiang et al., 2003).
Also, several antiviral compounds have been tried as therapeutic use in earlier decades(Kinchington et al., 1995).Nucleosidederivative drugs such as acyclovir (AVC), gancyclovir(GCV) and pencyclovir have been widely approved drugs for the treatment of HSV infections.However, widespreaduse of these drugs has shown resistance especially inimmuno compromised and bone marrow transplantrecipients. (Van denet al., 19986 and Vijayan et al., 2004). In order to circumvent the problem of viral resistance, development of new antiviral products with different mechanism of action is crucial. The activity of the Indian medicinal plant extract, Swertiachirata agains therpes simplex virus type-1 (HSV-1) using multipleapproaches.
Previously, EHP (1-(2-ethyl,6-heptyl)phenol) compound which extract from Cuminum cyminum (cumin) seeds by benzene solvent had possessed antifungal activity in an in vitro study against ten pathogenic fungal isolates (Mekawey et al., 2008). Also, high activities were recorded against and seven cell lines of tumor(Mekawey et al., 2009). The present study was designed to evaluate the antiviral and antidermatophyte activity of EHP (1-(2-ethyl,6-heptyl)phenol) compound, the antifungal drugs activities at the same concentrations and organic solvent are compared. The percentage of inhibition and MIC are also recorded.
Materials and Methods
Plant Material
The present study deals with the screening of 1-(2-Ethyl, 6-Heptyl) Phenol (EHP)extracted from seed of Cuminum cyminum (cumin) for anti-dermatophytic and antiviral activities.
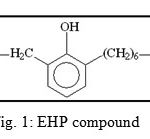 |
Figure 1: EHP compound
|
Fungal Specimen Collection
The specimens were collected from different parts of the body (skin scrapings, nails, hairs) of patients suffering from dermatophycoses. Forty (40) individuals of different ages, many childeren were sampled. This procedure was carried out using forty (40) new surgical blades for each individual. Specimens were collected by scraping affected spots into clean sheets of paper which were then transferred into sterile containers that had been properly labeled with respect to each individual’s data; these were brought to the laboratory for inoculation.
Identification of fungal isolates
An Image analysis system, soft imaging system GmbH software (analySIS ® pro ver. 3.0) at the fungal identification unit of The Regional Center for Mycology and Biotechnology AL- Azhar University was used for identifying the isolated dermatophytes.
Culture Media
Sabouraud Dextrose Agar (SDA) [Dextrose, 20.0g; Bacteriological Peptone, 10.0g; Agar, 20.0 g; 0.1 g/L cyclohexamide was used for isolation]. The pH of the media was adjusted at 5.6 ± 0.2 at 25 (±2) °C. Other media were used for maintenance of fungal isolates; Malt Extract Agar (MEA) [Malt extract, 20.0g; Bacteriological Peptone, 5.0g; Agar, 20.0g]. The pH was 5.4 ± 0.2 at 25 (±2)°C. Each medium was prepared by dissolving the solid ingredients in 1 liter of cold distilled water and then heating to 60-70 °C with stirring. Media were sterilized by autoclaving at 121°C (1.5 atm.) for 15-20 minutes (Atlas, 1993). For maintenance of stock cultures, 15 ml of cooled molten agar were poured into test tubes, auto claved at 121 °C for 15 minutes, and then tilted to provide slopes for stock cultures.
Screening of Antidermatophytic Activity
The filter paper disc method was used for screening the antifungal activity of EHP. Rinsed Petri plates were sterilized in an oven at 110oC overnight. Each of the sterilized plates was half filled with Sabouraud’s Dextrose Agar medium. The thickness of the agar medium was kept equal in all the Petriplates. Standard size Whatman filter paper discs (6.0 mm in diameter) were sterilized in an oven at 140oC for one hour, saturated with different concentrations of EHP and airdriedat room temperature under aseptic condition to remove any residual solventthat might interfere with the determination. The discswere then placed on the surface of sterilized Sabouraud’s Dextrose Agar Medium that had been inoculated with the investigated dermatophytes.
Each of the plates was homogenized to ensureuni form distribution of the inoculum and air dried to remove surface moisture. Petri plates containing thepaper discs (6 mm) dipped in benzene, methanol and water were then run ascontrol. Before incubation, all the test and control Petriplates were kept at 5oC for one hour to allow the diffusion of the substance from the disc into the agar medium plate. Plates were incubated at 37 oC for 48–72 hours, after which the zone of inhibition was measured. All the experiments were done in five replicates.
Determination of minimal inhibitory concentration of EHP compound on human pathogenic fungal growth:
Determination of the Minimum Inhibitory Concentration (MIC) of EHpcompound was carried out by broth dilution assay. Different standard discs (Griseofulvin; Itraconazole; Terbinafin, fluconazole and Ketoconazole) dissolved in methanol/dis. water for different fungi were used for determining the activity of EHP (compared to the highest standard value). Two-fold serial dilutions of the extracts were prepared in Sabouraud Dextrose broth to give concentrations ranging from 100- 1.56mg/ml. 0.2 ml of each of the microorganisms suspension was inoculated into the different concentrations of the test compound in test tubes. The tubes were incubated at 37±2 oC for 4-5 days. The concentration of the extract which exhibited no visible growth of the fungus was considered as the MIC.
Screening of Antiviral Activity
Determination of extract cytotoxicity
The ctotoxcity of EHP was performed according to(Van den Berghe et al., 1978) at the virology center , Faculty of medicine, Al-Azhar university.
Titration of HAV – H10, HSV – I, COX – B4 infectivity
Titration of HAV – H10, HSV – I, COX – B4 infectivity isolated was performed by plaque formation method according to (Dulbecco and Vogt, 1954). The virus infectivity titer represented as a number of plaque formation unit (P.F.U.) / ml of the stock virus suspension and it was calculated from the following equation:
P.F.U./ml = No. of plaque X reciprocal of dilution X reciprocal of volume in ml.
Determination of anti-infectivity effect of EHP
Anti-infectivity of EHP was achieved according to (Kaul et al., 1985).
Results
Evaluation of the Antifungal Activity of EHP
Table (1) and figure (2) shows the antidermatophytic activities of different EHP concentrations against the investigated dermatophytes. Trichophyton terrestre was the most resistant strain to EHP, exhibiting an inhibition zone of 3mm at 25 µg/ml, followed by Trichophyton schoenleiniiwith an inhibition zone of 4mm at 20µg/ml.
The most sensitive isolates wereE. floccosumand M. fulvum, each exhibiting an inhibition zone of 2mm at the least studied concentration of 2.5 µg/ml.
Thein vitro susceptibility of the clinical dermatophyteisolates against EHP and frequently used 5 antifungal agents was investigated (Table 2 and Figure 2).
MIC for EHP ranged from 2.5 to 25µg/ml. It exhibited the minimum MICvalue of 2.5µg/ml forE. floccosum and M. fulvum while the maximum one of 25µg/ml for T. terrestre.Table (2) and figure (2) reveals the competing capability of EHP regarding the investigated isolates with the exception of T. terrestre and T. schoenleinii as their MICs, 25 and 20 µg/ml respectively, greatly exceeded the cytotoxic concentration of EHP (10 µg/ml).
Table 1: Antidermatophytic activities of different concentrations of (EHP) (100 μl)
|
Fungal isplates
|
Zones of Inhibition (mm) of different concentration of cumin compound (EHP) (µg/ml) | |||||
| 25 | 20 | 15 | 10 | 5 | 2.5 | |
| Microsporum gypseum | 20 | 16 | 10 | 6 | – | – |
| Microsporum fulvum | 30 | 25 | 20 | 14 | 8 | 2 |
| Microsporum ferrugineum | 20 | 15 | 10 | 7 | 4 | – |
| Microsporum canis | 22 | 16 | 12 | 8 | 5 | – |
| Epidermophyton floccosum | 34 | 30 | 25 | 12 | 8 | 2 |
| Trichophyton interdigitale | 35 | 26 | 20 | 6 | – | – |
| Trichophyton mentagrophytes | 26 | 20 | 10 | 5 | – | – |
| Trichophyton schoenleinii | 10 | 4 | – | – | – | – |
| Trichophyton terrestre | 3 | – | – | – | – |
– |
| Trichophyton tonsurans | 29 | 15 | 8 | 3 | – | – |
Table 2: MICs (µg/mL)of EHP compared to antifungal drugs
|
|
EHP | Griseofulvin | Itraconazole | Terbinafin | fluconazole | Ketoconazole |
| M. gypseum | 8 | 6.25 | 2.5 | 3.125 | 3.125 | 3.125 |
| M. fulvum | 2.5 | 3.125 | 2.5 | 1.25 | 6.25 | 3.125 |
| M. ferrugineum | 5 | 6 | 2.5 | 2.25 | 5 | 3 |
| M. canis | 4 | 5 | 4 | 1 | 2 | 1.5 |
| E. floccosum | 2.5 | 3 | 1.5 | 1 | 5 | 2 |
| T. interdigitale | 7.5 | 5 | 6.5 | 2 | 6.25 | 3.5 |
| T. mentagrophytes | 10 | 6.25 | 2.5 | 1.5 | 17.5 | 1 |
| T. schoenleinii | 20 | 4 | 2 | 1 | 5 | 1.5 |
| T. terrestre | 25 | 7.5 | 4 | 2 | 10 | 3 |
| T. tonsurans | 10 | 5 | 7 | 1 | 9 | 1.7 |
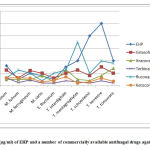 |
Figure 2: MIC (µg/ml) of EHP and a number of commercially available antifungal drugs against fungal pathogens.
|
Evaluation of the Antifungal Activity of EHP
Two EHP concentration, 6.25, and 12.5 ug/ml, were used toinvestigate its effect on viral activity using three widespread viruses (CoxB4, HSV1 and HAV). The percentage of viral plaque reduction was not greatly affected by EHP concentrations (figure 3).
Maximum activity was observed against HAV resulting in a plaque reduction percentage of 70 regarding both concentrations. HAV was followed by CoxB4 and then HSV1 with reduction percentages of 65 and 60, respectively.
No cytotoxicity was observed for EHP as ensured from the negative effect of EHP on vero cells (Fig. 3).
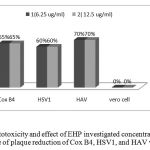 |
Figure 3: Cytotoxicity and effect of EHP investigated concentrations on the percentage of plaque reduction of Cox B4, HSV1, and HAV viruses.
|
Discussion
Compounds extracted from plants can provide an alternative approach to new therapies. They present characteristics such as high chemical diversity, lower cost of production and milder or inexistent side effects compared with conventional treatment (jardim et al., 2014).
In the current study, EHP, a compound extracted from cumin and was proved to possess antibacterial, antifungal and antitumor activities (Mekawy et al., 2007 & 2009), was found to possess antidermatophytic as well as antiviral activities.
Regarding dermatophytes, EHP exhibited activity against the ten investigated species; 5 species belonging to Tricophyton, 4 species to Microsporum, and one to Epidermophton, with E. flocossum and M. fulvum exhibiting inhibition zones at all the investigated concentrations even the lowest one (2.5µg/ml). According to the available research, a few studied the effect of cumin on dermatophytes; Romagnoli et al. (2010) studied the antidermatophytic and antifungal activity of essential oil from fruits of indian Cuminum cyminum which proved active in general on all investigated fungi but in particular on the dermatophytes, where Trichophytonrubrum was the most inhibited fungus also at the lowest dose of 5 microL.Other studies concerned the in vitro antifungal activities of essential oil from Cuminum cyminum, however against different pathogenic fungi, not dermatophytes; Naeini et al. (2014) studied the effect of essential oil from Cuminum cyminum on Candida strains where the results suggested the potential substitution of the antifungal chemicals by C. cyminum essential oil as natural inhibitors to control the growth of the most important pathogenic Candida species and alternative therapies for candidiasis.
Generally, the antimicrobial activity of essential oils of cumin seeds was reported by other investigators. Ozkan (2003) reported that essential oils of cumin potentially might be used as antibacterial agents to prevent the spoilage of food products. Iacobellis et al. (2005) also suggested using cumin oils to control bacterial diseases. Ani et al. (2006) reported the inhibitory effect of cumin extract on the growth of some food-borne pathogenic and spoilage bacteria. The antibacterial effect of cumin extract was also shown by Bonjar (2004) and Damasius et al. (2007).
The current study compared the MIC of EHP to that of a number of commercially available antidermatophtic agents against dermatophytic isolateswhere EHP proved efficient against all the investigated isolates except for T. terrestre and T. schoenleinii whose MICs exceeded the cytotoxic EHP concentration of 10 mg/ml. The current MIC results agreed with those ofIndira (2014) who reported that terbinafine had the lowest MIC range of 0.001 to 0.64 μg/ml followed by ketoconazole at a MIC range of 0.01-3.84 μg/ml. The itraconazole showed a MIC range of 0.082-20.45 μg/ml whereas the griseofulvin and fluconazole showed a highest MIC range of 0.32-5.12 μg/ml. Hence, in the current study, with the exception of T. terrestre and T. schoenleinii, EHP proved to compete with frequently-used, commercial antifungal agents
The present study also indicated antiviral activity of EHP against Cox B4, HSV1, and HAV, with HAV being the most affected followed byCox B4 and then HSV1.
According to the available research, only one studied the antiviral activity of cumin; Motamedifar et al. (2010) reported that the methanolic extract of cumin seeds has inhibitory effect on HSV-1. They also reported thatthe exact mechanism of cumin methanolic extract antiviral activity has not been studied yet and might be due to the interaction of some components of extract including phenolics with Vero cell membrane and/or HSV-1 envelope. This agrees and reinforces the current results as the structure of EHP [1-(2-Ethyl, 6-Heptyl) Phenol] possess a phenolic ring. Additionally, the concentrations used by Motamedifar et al. (0.01, 0.1, 0.25, 0.5 and 1 mg/ml) were much more than those used in the current study (6.25, and 12.5 µg/ml) which used the pure active compoundextracted from cumin seed extract. Other research concerned the activity of various plant extracts on viruses; e.g., Jardimet al. (2015) who reported that natural alkaloids andlignans isolated from Brazilian plants dramatically inhibited HCVreplication in vitro.
Conclusively, the antimicrobial and antiviral activity of the natural, purified, phenolic compound extracted from cumin seeds can be extended for future investigation and application into the field pharmacology, phytochemistry, or food chemistry for the development of better medicinal or preservative preparation.
References
- Abad Maria J.; Ansuategui, M. and Bermejo,P.(2007 ).Active antifungal substances from natural sources Issue in Honor of Prof. Atta-ur-Rahman ARKIVOC (vii) 116-145ISSN 1424-6376 Page 116 ©ARKAT
- Abonyi, D.O. (2009). In vitro antiviral effects of extracts from the lichenParmelia perlata ACH. (Family: Parmeliaceae) and Garcinia kolaHeckel (Family: Guttiferae) on some selected viruses. MSc dissertation submitted to the Department of Pharmaceutics,University of Nigeria, Nsukka.
- Aly, A.A.; Omar, S.A.; Zayed, S.M.E. and Mansour, M.T.M. (2000). Use of saponin – containing A triplex nummularia to suppress damping of cotton seedling. J. Agric. Sci. Mansura Uni., 25: 7621-7631.
- Aly, M.M. and Bafiel, S. (2008). Screening for antimicrobial activity of some medicinal plants in Saudi Arabia. World conference on medical and aromatic, 2008.
- Ani, V.; Varadaraj, M.C.and Akhilender, N. K. (2006). Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum). Eur Food Res Technol; 224: 109–15.
- Bonjar, S. (2004). Evaluation of antibacterial properties of some medicinal plants used in Iran. J Ethnopharmacology; 94: 301-5.
- Chermette, R.; Ferreiro, L.and Guillot, J. (2008). Dermatophytoses in animals. Mycopathologia, 166: 385-405.
- Chiang, L.C; Cheng, H.Y.; Liu, M.C.; Chiang, W. and Lin, C.C (2003).In vitroanti-herpes simplex viruses and anti adenovirus activity oftwelve traditionally used medicinal plants in Taiwan. BiolPharm Bull; 26:1600-4.
- Damašius, J.; Škėmaitė M.and Kirkilaitė, G. (2007). Antioxidant and antimicrobial properties of carway (Carum carvi.) and cumin (Cuminum cyminum) extracts. VeterinarijaIr Zootechnika; T. 40 (62).
- Desselberger, U. (1995) .Molecular Epidermiology, In: Medical Virology: A Practical Approach. Desselberger. U. (1st ed.) Oxford University Press,New York, pp. 173-190.
- Field, A.K and Biron, K.K. (1994) .The end of innocence. Revisited: Resistance of herpesviruses to antiviral drugs. Clin Microbiol Rev; 7:1-13.
- Iacobellis, N,S.;Lo Cantore, P.; Capasso. F.and Senatore, F. (2005) Antibacterial activity of Cuminumcyminum and Carum carvi esential oils. J Agric Food Chem; 53:57-61.
- Indira,G. (2014).In Vitro Antifungal Susceptibility Testing of 5 Antifungal Agents against Dermatophytic Species by CLSI (M38-A) Micro Dilution Method. Clin Microbial: Volume 3 • Issue 3.
- Jardim, A.C.G.; Igloi, Z.; Shimizu, J.F. ; Santos, V.A.F.F.M.; Felippe, L.G.; Mazzeu, B.F.; Amako, Y. ;Furlan, M.; Harris, M. and Rahal P. (2015): Natural compounds isolated from Brazilian plants are potent inhibitors of hepatitis C virus replication in vitro. Antiviral Research 115: 39–47.
- Kinchington, D.; Kangro, H. and Jeffries, K.J. (1995). Design and testing of antiviral compounds, In: Medical Virology: A Practical Approach.Desselberger, U. (ed) Oxford University Press, New York, pp. 147-171.
- Mekawey, A.A.I.; Mourad, M.; Farag R.M. and Mokhtar, M.M. (2008). Fungal Activity and Chemicalcomposition of seed extracts of Cuminumcyminum. Al-Azhar Bulletin of Science- Basicscience Sector. Proceeding of the 6th Al-AzharInternational Scientific Conference, 261-272.
- Mekawey, A.A.I; Mokhtar, M.M. and Farrag,R. M. (2009): Antitumor and Antibacterial Activities of [1-(2-Ethyl, 6-Heptyl) Phenol] from Cuminum cyminum Seeds. Journal of Applied Sciences Research, 5(11): 1881-1888.
- Motamedifar, M.; Ghafari, N and Shirazi, P.T. (2010). The Effect of Cumin Seed Extracts against Herpes Simplex Virus Type 1 in Vero Cell Culture. Iran J Med Sci; 35(4): 304-309.
- Naeini, A.; Jalayer Naderi, N. and Shokri,H. (2014): Analysis and in vitro anti-Candida antifungal activity of Cuminumcyminum and Salvadorapersica herbs extracts against pathogenic Candida strains. Journal of Medical Mycology. Volume 24, Issue 1, Pages 13–18.
- Ozkan, G.; Sagdiç, O. and Ozcan, M. (2003).Inhibition of Pathogenic Bacteria by Essential Oils at Different Concentrations. Food Science and Technology International; 9: 85-8.
- Romagnoli, C.1. ; Andreotti, E.; Maietti, S., Mahendra, R. and Mares, D. (2010): Antifungal activity of essential oil from fruits of Indian Cuminum cyminum. Pharm Biol. Jul; 48(7):834-8.
- Soylu, E, M; Tok, F.M; Soylu, S.; Kaya, A.D. and Evrendilek, G.A.( 2005). Antifungal activities of essential oils on post-harvest disease agent Penicilliumdigitatum. Pak. J. Biol. Sci., 8: 25-29.
- Vanden, D.A.; Vlietinck, A.J. and Van Hoof, D.L. (1986).Plant products aspotential antiviral agents. Bull Inst Pasteur; 84:101-47.
- Vijayan, P.; Raghu, C.; Ashok, G.; Dhanaraj, S.A. and Suresh, B. (2004).Antiviral activity of medicinal plants of Nilgiris. Indian J Med Res; 120:24-9.
- Yoshida, M.; Fuchigami, M.; Nagao, T.; Okabe, H.; Matsunaga, K.,Takata, J., Karube, Y.; Tsuchihashi, R.; Kinjo, J.; Mihashi, K. Fujioka,T.(2005).
- Antiproliferative constituents from Umbelliferae plants VII. Active triterpenes and rosmarinic acid from Centella asiatica. Biol. Pharmacol. Bull., 28: 173-175.







