Manuscript accepted on :December 15, 2008
Published online on: --
Plagiarism Check: Yes
Anna Balaji1*, V. P. Pandey2, R. Manavalan2 and M. S. Srinath3
1Department of Pharmaceutics, Dayananda Sagar College of Pharmacy, Bangalore (India).
2Department of Pharmacy, Annamalai University, Annamalainagar (India).
3Department of Pharmacy, CMR College of Pharmacy, Bangalore (India).
Abstract
Our study reported herein utilized the Agrobacterium tumefaciens–induced potato disc tumor assay. The objective was to detect of extent of antineoplastic activity in the potato disc tumor induction assay by Cisplatin, its HP-b-CD complexes and gelatin nanoparticles loaded with Cisplatin and its HP-b-CD complexes. Tumor inhibition was seen in all the tested drugs and its formulations. The order of activity was: 1:2 Cisplatin/HP-b-CD > 1:1 Cisplatin/HP-b-CD > Cisplatin > 1:2 Cisplatin/HP-b-CD loaded nanoparticles >1:1 Cisplatin/HP-b-CD loaded nanoparticles > Cisplatin loaded nanoparticles. The A. tumefaciens–induced potato disc tumor assay was an effective indicator of the extent of antitumor activity regardless of the mechanism of drug action. In the brine shrimp lethality bioassay (BSLT), the Medium Lethal Concentrations (LC50 value) of Cisplatin and Cisplatin/HP-b-CD inclusion complexes were determined using Artemia salina L. (Artemiidae) with the objective was to compare the LC50 values of the pure drug, its HP-b-CD complexes and loaded gelatin nanoparticles to determine their relative degree of cytotoxicity. The bioassay results showed significant dose dependent mortality. Cisplatin, 1:1 Cisplatin/HP-b-CD and 1:2 Cisplatin/HP-b-CD inclusion complexes, Cisplatin loaded nanoparticles, 1:1 Cisplatin/HP-b-CD loaded nanoparticles and 1:2 Cisplatin/HP-b-CD loaded nanoparticles exhibited potent brine shrimp lethality with LC50 of 106.71, 83.43, 76.53, 270.96, 177.59 and 149.55 µg/mL respectively. Thus, potato disc assay would be acceptable as a primary general screen for antineoplastic activity of various complexes of Cisplatin and its formulations regardless of mode of inhibitory action on tumor formation. The present study also supports that brine shrimp bioassay is simple, rapid, reliable and convenient method for assessment of degree of cytotoxicity.
Keywords
Cisplatin; Cisplatin/Hydroxypropyl-b-Cyclodextrin inclusion complex; antitumor; cytotoxic
Download this article as:| Copy the following to cite this article: Balaji A, Pandey V. P, MBalaji A, Pandey V. P, Manavalan R, Srinath M. S. Cytotoxic Evaluation of Cisplatin, Cisplatin/Hydroxypropyl- b-Cyclodextrin Complexes and their Nanoparticles Drug Delivery Systems.Biomed Pharmacol J.2008;1(2).anavalan R, Srinath M. S. Cytotoxic Evaluation of Cisplatin, Cisplatin/Hydroxypropyl- b-Cyclodextrin Complexes and their Nanoparticles Drug Delivery Systems. Biomed. Pharmacol. J.;1(1) |
| Copy the following to cite this URL: Balaji A, Pandey V. P, Manavalan R, Srinath M. S. Cytotoxic Evaluation of Cisplatin, Cisplatin/Hydroxypropyl- b-Cyclodextrin Complexes and their Nanoparticles Drug Delivery Systems.Biomed Pharmacol J. 2008;1(2). Available from: http://biomedpharmajournal.org/?p=450 |
Introduction
Cis-Diaminedichloroplatinum (II) (Cisplatin) is a major antineoplastic drug widely used in the treatment of several malignancies1,2. It has been widely accepted because of its potent cytotoxic effects on a variety of tumor types including testicular, ovarian, and cervical carcinoma3. Although Cisplatin is one of the most potent anticancer agents available for the treatment of solid tumors4, its use is associated with serious side effects including renal toxicity, and late neurotoxicity as well as ototoxicity5,6,7. 20% of patients receiving high-dose Cisplatin have severe renal dysfunction. Although various measures such as aggressive hydration prior to Cisplatin administration have helped reduce the incidence of certain toxicities such as nephrotoxicity, prolonged courses of Cisplatin at high doses still predispose patients to other adverse effects such as neuropathy5,6,7.
One of the most efficient and rational approach to overcome the limitation of toxicity of Cisplatin in nontarget tissues is to design a drug delivery system with selective tumor targeting and controlled releasing properties. In particular many nanosized polymers and liposomes are recently known to show excellent tumor targeting properties with enhanced permeability and retention (EPR) effect 8,9,10,11,12,13. The requirements of an ideal targeting system are i) biocompatibility, biodegradability, and low antigenicity, ii) protection of the drug, iii) maintenance of the integrity till the target is reached, iv) avoidance of side effects, v) membrane passage, vi) target recognition and association, vii) controlled drug release, and viii) elimination upon drug release14.
Gelatin is a proteinaceous natural polymer which has been successfully used as a nanomaterial. It has the added advantage of being able to undergo chemical modifications via its amino acid residues before and after the preparation of nanoparticles15. Gelatin is known for its good biodegradability, biocompatibility16,17, as well as its low immunogenicity18, 19 which made gelatin as “Generally Recognized as Safe (GRAS) substance in the area of food additives by the U.S. Food and Drug Administration (FDA)*
Crown gall is a neoplastic disease of plants which is induced by the gram negative bacteria Agarbacterium tumefaciens20,21, first reported by Smith and Townsend22 and Jensen23. This disease is characterized by a mass of tissue bulging from stems and roots of woody and herbaceous plants is produced. These masses (tumors) may be spongy or hard, and may or may not have a deleterious effect on the plant. The tumors produced are histologically similar to those tumors found in humans and animals24. The bacteria possess large Ti (tumor inducing) plasmids which carry genetic information (T DNA), that transform normal / wounded plant cells into autonomous tumor cells25. Following tumor induction, the autonomous proliferation of the tumor cells becomes entirely independent of the bacteria26. The mechanism of tumor induction is similar to that in animals27,28,29. The Ti-plasmid causes the plant’s cells to multiply rapidly without going through apoptosis, resulting in tumor formation similar in nucleic acid content and histology to human and animal cancers24.
The results suggest that the potato disc assay is a safe, simple rapid and inexpensive in-house screen for 3PS (in vivo, mouse leukemia) antitumor activity. It is statistically more predictive of 3PS30,31,32 (P 388 leukemia) activity than either the 9KB (human nasopharyngeal carcinoma) or 9PS (murine leukemia) cytotoxic assays. The assay also gives indication of tumor-promoting or carcinogenic properties of the test samples.
Artemia salina L. (Artemiidae), the brine shrimp larva, is an invertebrate used in the alternative test to determine toxicity of chemical and natural products. The common brine shrimp (artemia) belongs to phylum Arthropoda, class Crustacea. The artemia life cycle begins by the hatching of dormant cysts, which are encased embryos that are metabolically inactive. The cysts can remain dormant for many years as long as they are kept dry. When the cysts are placed back in salt water they are rehydrated and resume their development. They can tolerate high concentrations of salt in seawater, which varies from 2.9% to 3.5%. Adult brine shrimp can tolerate a salt content of as high as 50%. They are non selective with respect to their food and live entirely on the photosynthetic green alga (singular of algae) Dunaliella. Like many other primitive aquatic plants, this organism is attracted to light, rising to the surface in the daytime, and sinking at night. The positive phototaxis of Artemia keeps it at the same depth as its prey.
In order to study the degree of cytotoxicity of Cisplatin, 1:1 Cisplatin/HP-β-CD complex , 1:2 Cisplatin/HP-β-CD complex and gelatin nanoparticles loaded with the aforementioned pure drug and complexes, we performed BSLT, which is based on the ability to kill laboratory cultured brine shrimp (Artemia nauplii). The brine shrimp assay was proposed by Michael et al33., and latter developed by Vanhaecke et al34 and Sleet and Brendel35. The assay is considered a useful tool for preliminary assessment of toxicity and it has been used for the detection of fungal toxins, plant extract toxicity, heavy metals, pesticides and cytotoxicity testing of dental materials36.37,38,39,40. This method is very useful tool for the isolation of bioactive compounds from plant extracts41.
The brine shrimp lethality bioassay is an efficient, rapid and inexpensive test that requires relatively a small amount of sample (2–20 mg). This bioassay has a good correlation with cytotoxic activity in some human solid tumors and with pesticidal activity, and has led to the discovery of the annonaceous acetogenins as a new class of natural pesticides and active antitumoral agents42.
Cisplatin was complexed with Hydroxypropyl-β-Cyclodextrin with a view to improve its stability, water solubility, bioavailability and possible decrease in its undesirable side effects. The objective of the present study was to evaluate the degree of cytotoxicity of Cisplatin, its complexes with HP-β-CD and gelatin nanoparticles loaded with Cisplatin and HP-β-CD complexes using potato disc test and brine shrimp lethality bioassay
The development of simple in vitro bioassays like potato disc test and BSLT could offer numerous advantages as alternatives to expensive animal testing in initial studies. Also, it was attempted to show that both assays are simple, rapid, reliable and convenient method for assessment of cytotoxic studies of synthetic compounds.
Materials and Methods
Materials
Cisplatin from Cipla, India and Brine shrimp eggs (Artemia salina L.) from FRLHT, Bangalore, India were obtained as gift samples. Gelatin Type A (175 bloom) having molecular weight between 40 kDa and 50 kDa was purchased Sigma Co., USA. Glutaraldehyde 25% w/w in solution, Loba Chemie Pvt. Ltd., India; HPLC grade water, Qualigens Fine chemicals India; HP-β-CD (Mol. Wt. 1380), HiMedia, India; Agrobacterium tumefaciens, MTCC, Chandigarh, India were purchased. All solvents and chemicals were used of analytical grade. All products and chemicals were used as received from the manufacturers.
Preparation of Cisplatin – Hydroxypropyl-β-Cyclodextrin Complexes
The inclusion complex was prepared by freeze drying a solution of Cisplatin and Hydroxypropyl-β-Cyclodextrin in different molar ratios (1:1and 1:2). In brief Cisplatin and HP-β-CD were dissolved in HPLC grade water and was sonicated in an ultrasonic bath for 1 h at 25.0 ± 0.1 °C. The solution was later filtered through a 0.22 μM filter and the filtrate was frozen at – 40 °C and then freeze-dried at −70 °C for 24 h.
Preparation of drug loaded gelatin nanoparticles by two-step desolvation
Gelatin nanoparticles were prepared by a two-step desolvation method developed by Coester et al. 8. 25 mL of 5% gelatin type A (Bloom175) solution was prepared at room temperature (25 °C) with 25mg of drug (Cisplatin, 1:1 Cisplatin/ HP-β-CD complex and 1:1 Cisplatin/ HP-β-CD complex ) in it. Gelatin was desolvated by drop wise addition of an equal volume of acetone, a non-solvent for gelatin and kept for sedimentation. The supernatant was discarded and the sediment was dissolved in water and redesolvated at pH 2.5 with 50 mL of acetone with stirring (500 rpm). Gelatin particles were then cross-linked with 200μL of 25 % glutaraldehyde, the excess of which was neutralized by adding cysteine (500 mg), and finally purification was done by a three-fold centrifugation (16000 g for 20 min) and redispersion in acetone/water mixture (30/70). The purified nanoparticles were stored as dispersion in highly purified water (conductivity < 0.04μs/cm) at 4-8 °C.
Potato disc bioassay
Agrobacterium tumefaciens (strain MTCC 431), which carries the Ti (tumor inducing) plasmid, was cultured as described by McLaughlin38. The samples were prepared by dissolving 1.0 mg of compounds in 1 ml of 0.9 % w/v NaCl solution and subsequently diluted to 0.01, 0.001 and 0.0001 mg/L. This procedure is adapted from Coker, P.S. et al, with modifications taken from Ferrigni, N. R. et al43. Controls were prepared with pure Cisplatin. A minimum of three Petri dishes were used for each test and for the control. Potato discs were placed on solid agar-water medium in Petri dishes. Using a sterile micropipette, 400 uL bacteria solution (48 h culture of approx. 1 x 109 CFU/ml, as determined by an absorbance value of 0.96 ± 0.02 at 600 nm) was mixed with 400 uL of the appropriate test or control solution in Eppendorf tubes. 50 uL of the so prepared solution were applied to each potato disc. Control discs were prepared in the same way omitting the test substances. The Petri dishes were kept in the dark at 22°C for 14 days. The number of tumors was counted at the end of the incubation period. To determine the number of tumors, the potato discs were stained with a solution of I2(1 g)/KI (2 g) in 300 ml distilled H2O for 20 min and then destained in ethyl alcohol (95%) for 5 min. The tumors were counted using a dissecting microscope and their numbers were compared with controls. The inhibitory effect was evaluated on the basis of the comparative analysis among the mean values of the number of tumors in treated and untreated disks. Inhibition percentage of over 20% was considered to be significant.
Brine shrimp lethality test
The method described by Meyer et al45, (1982), was used in this assay. The brine shrimp were hatched from encysted eggs in a small rectangular two compartment steel tank (l4x6x8 cm) fabricated especially for culturing brine shrimps. Around 50-100 mL of artificial sea water (pH 9.0 adjusted with Sodium carbonate) is added and 10mg of eggs was sprinkled on top of the water and incubated for 48 h under constant aeration and illuminated with a tungsten filament lamp. After hatching, nauplii were separated from the shells and remaining cysts using a Pasteur pipette and transferred to fresh sea water. This was facilitated by attracting the shrimps to one side of the brighter compartment of the tank used for hatching with a light source. 20 organisms / 4.5 mL were pipetted into each of multi-welled culture plates using a Pasteur pipette. A stock solution of 100 µg/mL of pure drug and complexes of Cisplatin with Hydroxypropyl-β-Cyclodextrin were prepared in 0.9% normal saline. Pure drug and its complexes were tested at initial concentration of 1.0, 100 and 1000 µg/mL in each of three replicates with each well having a final volume of 5mL. They are then incubated at a temperature of 25°C for 24 h under direct light.
Lethality concentration determination
Counting for the chronic LC50 begins 24 h. after initiation of the tests. Survivors were counted after 24 h by examining under dissection microscope (10 x magnifications). The percentage of deaths at each dose was determined by comparing the mean surviving numbers in the test and control wells. LC50 values were obtained from the best-fit line plotted concentration verses percentage lethality. Cisplatin was used as a positive control in the bioassay.
Statistical analysis
The percentage lethality was calculated from the mean survival numbers in sample and control wells. LC50 values were obtained by best-fit line method. The data was processed using AnalystSoft, StatPlus – statistical analysis program, Version 2007, to estimate LC50 (Lethal concentration) values for statistically significant comparison of potencies.
Results and Discussion
For the potato disc assay, the results are expressed as percentages of the number of tumors on the control discs; inhibition is expressed as a negative percentage and stimulation is expressed as a positive percentage. Activity was deemed significant when two or more independent assays gave consistent negative values, indicating inhibition above 20%. The antitumor activity of Cisplatin, 1:1 Cisplatin/HP-β-CD complex , 1:2 Cisplatin/HP-β-CD complex and gelatin nanoparticles crosslinked with glutaraldehyde and loaded with the aforementioned pure drug and complexes was studied using the potato disc bioassay technique. The solvent used in potato disc test was 0.9 % NaCl solution and therefore the need to study the effect of solvent induced tumor induction was obviated. Cisplatin served as a positive control and inhibited tumor production in all concentrations.
There was significant difference in activity between Cisplatin and Cisplatin/HP-β-CD inclusion complexes at 0.01 mg/mL (Table 1.). HP-β-CD inclusion complexes of Cisplatin were more active at 0.01 mg/mL than Cisplatin at the same concentration. 1:2 Cisplatin/HP-β-CD complex was slightly more inhibiting than 1:1 Cisplatin/HP-β-CD complex at 0.01mg/mL. The Cisplatin loaded nanoparticles and Cisplatin/HP-β-CD complexes (1:1 and 1:2 ratios) loaded nanoparticles showed lesser inhibition than their respective pure drugs at all concentrations indicating a possible decrease in concentration of the drug available at the site of inhibition. This may be explained as follows. Of the total drug loaded into the nanoparticles, a part of the drug is entrapped within the matrix of the gelatin nanoparticles crosslinked by glutaraldehyde and a part of the drug is bound chemically to the amino acid residues present in the native gelatin44. The A. tumefaciens–induced potato disc tumor assay was an effective indicator of the extent of antitumor activity regardless of the mechanism of drug action.
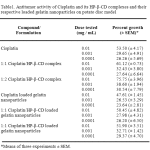 |
Table 1: Antitumor activity of Cisplatin and its HP-β-CD complexes and their respective loaded gelatin nanoparticles on potato disc model.
|
The brine shrimp lethality of Cisplatin, 1:1 Cisplatin/HP-β-CD complex , 1:2 Cisplatin/HP-β-CD complex and gelatin nanoparticles crosslinked with glutaraldehyde and loaded with the aforementioned pure drug and complexes to brine shrimp was determined using the procedure of Meyer et al45. The LC50 values obtained are given in Table 2. The degree of lethality was found to be directly proportional to the concentration. 1:2 Cisplatin/HP-β-CD complex showed highest cytotoxicity followed by 1:1 Cisplatin/HP-β-CD complex and Cisplatin. The LC50 values were obtained by a plot of percentage of the shrimp nauplii killed against the concentrations used and the best-fit line was obtained from the data by means of regression analysis.
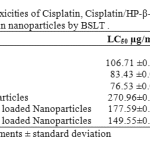 |
Table 2: Comparative toxicities of Cisplatin, Cisplatin/HP-β-CD complexes and their respective loaded gelatin nanoparticles by BSLT .
|
Unlike the Cisplatin and its HP-β-CD complexes, the loaded nanoparticles showed lesser cytotoxicity due to the drug entrapment and conjugation within matrix of the nanoparticles making it less available comparatively. The release of the drug is sustained over a long period of time (data not shown). The drug release kinetics from gelatin nanoparticles depends on the rate of water uptake, drug dissolution /diffusion rate and the polymer glass-rubbery transition including matrix erosion/degradation rate46. At higher temperature, the gelatin molecules have higher mobility and lower degree of crosslinking, which increases the swelling degree of the hydrogel nanoparticles. Also the water content is affected by the degree of crosslinking of the gelatin nanoparticles. Degradation of the nanoparticles occurs either after phagocytosis or by extracellular protease acting at either neutral or acidic pH. Lower toxicity of the nanoparticles may be attributed to the prevailing pH (pH 9.0) condition of the test.
Cisplatin/HP-β-CD complexes loaded nanoparticles showed greater cytotoxicity than Cisplatin loaded gelatin nanoparticles due to rapid release of the drug from the matrix of the nanoparticles than that of nanoparticles loaded with Cisplatin alone.
Gelatin nanoparticles formed from loading of Cisplatin/HP-β-CD complexes were smaller in size (data not shown) due to the presence of HP-β-CD compared to Cisplatin loaded nanoparticles and offered greater surface area and swelling profile which promoted rapid drug release. The release of Cisplatin from the nanoparticles is strongly affected by the swelling of the nanoparticles. Enhanced release of Cisplatin from the nanoparticles loaded with Cisplatin/HP-β-CD complex could be attributed to the difference in degree of hydration and subsequent swelling of nanoparticles.
On the other hand HP-β-CD complexes of Cisplatin showed highest cytotoxic effect due to greater water solubility of the complexes and enhanced penetration when compared with the pure drug. It has been widely believed that drug availability in cyclodextrin-containing formulations will be hampered by the slow release of drug molecules from the cyclodextrin cavities. However, it has been shown that the rates for formation and dissociation of drug/cyclodextrin complexes are very close to diffusion controlled limits with complexes being continually formed and broken down47.
Consequently, presence of water-soluble Cisplatin/HP-β-CD complexes right at the hydrated epithelial surface will frequently increase the availability of dissolved drug molecules, especially of lipophilic drugs with poor aqueous solubility48.
For years, cyclodextrins have been proposed in the formulation of poorly water soluble drugs, with the main objective of increasing their water solubility in order to possibly increase their bioavailability. Cyclodextrins have hydrophobic cavity and hydrophilic external surface. Poorly soluble drug accommodates in hydrophobic cavity. This molecular entrapment improves drug solubility. The cyclodextrin complexes of hydrophobic drugs dissolve faster and better than pure drug49,50.
The aqueous solubility of α, β and γ cyclodextrin is much lower than that of comparable linear dextrins, most probably due to relatively strong binding of the cyclodextrin molecules in the crystal state (i.e. relatively high crystal energy).In addition, β – Cyclodextrin molecules form intramolecular hydrogen bonds that diminish their ability to form hydrogen bonds with the surrounding water molecules. Hydroxypropyl-β-Cyclodextrin is a water-soluble cellulose derivatives synthesized and were used in large quantities in a variety of industrial products. Conjugation of Cisplatin with Hydroxypropyl-β-Cyclodextrin results in a significant increase in drug solubility thereby increasing its cytotoxic activity evident by the decreased LC50 values.
Conclusion
As there is a limit to the usage of laboratory animals in toxicological tests51, the potato disc test and brine shrimp lethality test, which are sensitive to a variety of compounds, can be effectively utilized. Based on the results obtained, we suggest that the both bioassays afford a convenient, simple, and inexpensive avenue for determining the relative toxicity of natural and synthetic compounds which in most cases correlates reasonably well with cytotoxic and anti-tumor properties38. These tests are quick, and allows a great number of samples to be tested and processed adequately52.
In conclusion we affirm that there is an increase in cytotoxicity of the Hydroxypropyl-β-Cyclodextrin complexes of Cisplatin due to increase in solubility. The drug loaded nanoparticles showed decreased toxicity and could successfully increase drug concentration in cancer tissues and also act at cellular levels, enhancing antitumor efficacy. The potato disc test and brine shrimp lethality bioassay are very convenient and effective methods for assessment of cytotoxic studies of synthetic compounds
References
- A.W. Prestayko, S.T. Crooke, S.K. Carter, Cisplatin: Current Status and New Developments, Academic, New York, (1980).
- P.J. Loehrer, L.H. Einhorn, Drugs five years later. Cisplatin, Ann. Intern. Med. 100 (5) 704– 713, (1984).
- Bonetti, A., Franceschi, T., Apostolo, P., Messori, A., Sperotto, L., Cetto, G. L., et al. Cisplatin pharmacokinetics using a five-day Schedule during repeated courses of chemotherapy in germ cell tumors. Ther Drug Mon, 17, 25– 32, (1995).
- Y. Sedletska, M.J. Giraud-Panis, J.M. Malinge, Cisplatin is a DNA-damaging antitumour compound triggering multifunctional biochemical responses in cancer cells: importance of apoptotic pathways, Curr. Med. Chem. Anticancer Agents 5 251–265, (2005).
- Judson I, Kelland LR. New developments and approaches in the platinum arena. Drugs, Jun; 59 Suppl. 4: 29-36, (2000).
- Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of Cisplatin and carboplatin. J Clin Oncol, Jan; 17 (1): 409-22, (1999).
- Huitema ADR, Smits KD, Mathot RA, et al. The clinical pharmacology of alkylating agents in high-dose chemotherapy. Anticancer Drugs. Aug; 11 (7): 515-33 (2000).
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura, K. Hori K, J. Control. Release 65 271–284, (2000).
- H. Maeda, Advan. Enzyme Regul. 41, 189–207, (2001).
- H. Maeda, T. Sawa, T. Konno, J. Control. Release, 74 , 47–61, (2001).
- T. Minko, P. Kopeckova, J. Kopecek, Int. J. Cancer, 86, 108– 117, (2000).
- J. Kopecek, P. Kopeckova, T. Minko, Z.-R. Lu, C.M. Peterson, J. Control. Release, 74, 147–158, (2001).
- R. Duncan, Pharm. Sci. Technol. Today 2, 441–449, (1999).
- Scholes, P. D., Coombes, A. G. A., Davies, M. C., Illum, L., and Davis, S. S.;
- Particle engineering of biodegradable colloids for site-specific drug delivery; Controlled Drug Delivery, 17, 73-106, (1997).
- Djagny, K. B., Wang, Z., and Xu, S.; Gelatin: A valuable protein for food and pharmaceutical industries: Review; Critical Reviews in Food Science and Nutrition, 41(6), 481-492, (2001).
- Yamamoto, M., Ikada, Y., and Tabata, Y.; Controlled release of growth factors based on biodegradation of gelatin hydrogel; Journal of Biomaterials Science.Polymer edition, 12(1), 77-88, 2001.
- Stevens, K. R., Einerson, N. J., Burmania, J. A., and Kao, W. J.; In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks; Journal of Biomaterials Science, Polymer Edition, 13(12), 1353-1366, (2002).
- Schwick, H. G. and Heide, K.; Immunochemistry and immunology of collagen and gelatin; Bibliotheca Haematologica (Basel), 33, 111-125, (1969).
- Kuijpers, A. J., Engbers, G. H., Krijgsveld, J., Zaat, S. A., Dankert, J., and Feijen, J.; Cross-linking and characterisation of gelatin matrices for biomedical applications; Journal of Biomaterials Science. Polymer edition, 11(3), 225-243, (2000).
- Kahl G, Schell JS, Molecular Biology of Plant Tumors. Academic Press. NY, (1982).
- Lippincott JA, Lippincott BB, The genus Agrobacterium and plant tumorigenesis. Ann Rev Microbiol, 29:377–405, (1975).
- Smith, E. F.; Townsend, C. O. A plant-tumor of bacterial origin. Science, 25: 671–673, (1907).
- Jensen, C. O. Undersøgelser vedrørende nogle svulstlignende dannelser hos planter. Kgl. Veterinaer-Landbohøjskoles. Aarsskrift, 2: 91–143, (1918).
- Agrios GN, Plant diseases caused by prokaryotes: bacteria and mollicutes. Plant Pathology. Academic Press, San Diego. pp 407–470, (1997).
- Chilton, M. D.; Saiki, R. K.; Yadav, N.; Gordon, M. P.; Quetier, F. T. DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proceedings of the National Academy of Sciences USA, 77: 4060–4064, (1980).
- Zaenen, I.; van Larekehe, N.; Teuchy, H.; van Montagu, M.; Schell, J. Sueprcoiled circular DNA in crown-gall inducing Agrobacterium strains. Journal of Molecular Biology, 86: 109–127, (1974).
- Becker FF, Cancer. A Comprehensive Treatise. Etiology: Viral Carcinogenesis. Plenum Press, NY, Vol 2, (1975).
- Braun AC, The relevance of plant tumor systems to an understanding of the basic cellular mechanisms underlying tumorigenesis. Prog Exp Tumor Res 15: 165–187, (1972).
- Karpas A, Viruses and leukemias. Am Sci 70: 277–285, (1982).
- Galsky AG, Wilsey JP, Powell RG, Crown gall tumor disc bioassay: a possible aid in the detection of compounds with antitumor activity. Plant Physiol, 65: 184–185, (1980).
- Galsky AG, Kozimer R, Piotrowski D, Powell RG, The crown-gall potato disc bioassay as a preliminary screen for compounds with antitumor activity. J Natl Can Inst 67: 689–692, (1981).
- Ferigni NR, Putnam JE, Anderson B, Jacobsen LB, Nichols DE, Moore DS, McLaughlin JL, Powell PG, Smith CR Jr, Modification and evaluation of the potato disc assay and antitumor screening of Euphorbiacae seeds. J Nat Prod, 45: 679–686, (1982).
- Michael, A. S., Thompson, C. G., and Abramovitz, M. Artemia salina as a test organism for a bioassay. Science, 123: 464.
- Vanhaecke, P., Persoone, G., Claus, C., and Sorgeloos, P. 1981. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicologyl and Environmental Safety, 5: 382-387, (1956).
- Sleet, R. B. and Brendel, K. Improved methods for harvesting and counting synchronous populations of Artemia nauplii for use in developmental toxicology. Ecotoxicologyl and Environmental Safety, 7: 435-446, (1983).
- Harwing, J. and Scott, P. Brine shrimp (Artemia nauplii L.) larvae as a screening system for fungal toxins. Applied Microbiology, 21: 1011-1016, (1971).
- McLaughlin, J. L., Chang, C. J., and Smith, D. L. Bench-top bioassays for the discovery of bioactive natural products: an update. In: Rhaman, A. U. (Ed.), Studies in Natural Products Chemistry. Elsevier, 383-409, (1991).
- Martinez, M., Del ramo, J., Torreblanca, A., and Diaz-Mayans, J. Effect of cadmium exposure on zink levels in the brine shrimp Artemia partenogenitica. Aquaculture, 172: 315-325, (1998).
- Barahona, M. V. and Sanchez-Fortun, S. Toxicity of Carbamates to the Brine Shrimp Artemia salina and the Effect of Atropine, BW284c51, iso-OMPA and 2-PAM on Carbaryl Toxicity. Environmental Pollution, 104:469-476, (1999).
- Pelka, M., Danzl, C., Distler, W., and Petschelt, A. A new screening test of dental materials. Journal of Dentology, 28: 341-345, (2000).
- Sam, T. W. Toxicity testing using the brine shrimp: Artemia salina. In: Colegate, S. M. and Molyneux, R. J. (Eds.), Bioactive Natural Products Detection,Isolation, and Structural Determination. CRC Press, Boca Raton, FL: 442-456, (1993).
- McLaughlin JL, Rogers LL, Anderson JE. The Use of Biological Assays to Evaluate Botanicals. Drug Information Journal, 32: 513-524, (1998).
- Coker, P.S. et al, Phytomed, 2003, 133-138 with modifications taken from Ferrigni, N. R. et al, Journal of Natural Products, 45 (6), 679 – 686, 1982.
- Doxorubicin-loaded gelatin nanoparticles stabilized by glutaraldehyde: Involvement of the drug in the cross-linking process, Eliana Leo, Maria Angela Vandelli, Riccardo Cameroni, Flavio Forni, Intl J. Pharmaceutics 155: 75–82, (1997).
- Meyer, B. N., Ferrigni, N. R., Putnam, J. E., Jacobson, L. B., Nichols, D. E., and McLaughlin, J. L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica, 45: 31-34, (1982).
- C.S. Brazel, N.A. Peppas, Modelling of drug release from swellable polymers, Eur. J. Pharm. Biopharm. 49: 47–58, (2000).
- Stella,V.J., Rajewski, R.A. Cyclodextrins: their future in drug formulation and delivery. Pharm. Res. 14, 556–567, (1997).
- Loftsson,T.,Konr´aðsd´ottir, F.,M´asson, M. Influence of aqueous diffusion layer on passive drug diffusion from aqueous cyclodextrin solutions through biological membranes. Pharmazie, 61, 83–89, (2006).
- Belgamwar V S, Nakhat P D, Indurwade N H et al; Studies on occlusion complexes of furazolidone with cyclodextrins and their hydroxy propyl derivatives, Indian Drugs, 38(9), 479-482, 2001.
- M Narender Reddy, Tasneem R, Ramkarishna K et al; β-cyclodextrin complexes of celecoxib: Molecular modeling, characterization and dissolution studies, AAPS Pharm Sci, 6(1) 7, 1-9, (2004).
- Yajes R.: Animal ethics committees and the implementation of the Three Rs. Animal Alternatives, Welfare and Ethics. Edited by LFM Van Zutphen and M. Balls Elsevier Developments in Animal and Veterinary Sciences: 367–71, (1997).
- Ohno, Y., Kaneko, T., Inove, T., Morikawa, T., Yoshida, A., Fujii, A., Masuda, M., Ohno, T., Hayashi, M., Mumma, J., Akiyama, J., Hagaki, H., Ohkoshi, K., Okumura, H., Kakishima, H., Kasai, Y., Kurishita, A., Kujima, H., Saijo, K., Sakamoto, K., Sugiura, S., Sunouchi, M., Takano, K., Tatsumi, H., Tani, N., Chiba, K., Nakamura, T., Matsukawa, K. and Matsushige, C.: Interlaboratory validation of alternative methods to the eye irritation test for the safety evaluation of cosmetic ingredients: an overview of the plan and the results. Animal Alternatives, Welfare and Ethics. 27: 1155–8, (1997).







