Manuscript accepted on :
Published online on: 08-01-2016
Plagiarism Check: Yes
Umasudhakar1, T. Ramakrishnan2, Rekha2, Bhagya Meena1, Kamal Kannadasan3, Tamizhselvan4
1Department of Periodontics., Thai moogambigai dental college, Golden George nagar, Chennai 600107. 2Department of periodontics., Adhi parasakthi dental college, Melmaruvathur. Tamil nadu 3Department of oral and maxillofacial surgery Thai moogambigai dental college, Golden George nagar, Chennai 600107. 4Department Of Oral Pathology., Sree Ramachandra dental college, Chennai,
DOI : https://dx.doi.org/10.13005/bpj/816
Abstract
Chronic Periodontitis, an inflammatory disease caused by oral bacteria stimulates the host cells, neutrophils which release Reactive Oxygen Species(ROS) as a part of immune response. Diabetes Mellitus is a complex disease with varying degrees of systemic and oral complications. The study population consisted of 40 subjects belonging to both sexes were randomly selected. Subjects were divided into two groups, Chronic Periodontitis(CP) ( Group I) and Chronic Periodontitis with Diabetes Mellitus (CP-DM) (Group II).GCF, saliva and plasma were collected in all the two groups to estimate the Reactive oxygen metabolites levels. The values obtained in GCF were significant[ pvalue 0.001]and the ROM levels in saliva and plasma were significantly higher in Chronic Periodontitis with Diabetes Mellitus group compared to Chronic Periodontitis [ pvalue < 0.001] .The results of our study suggested that a significant oxidative stress may occur in Chronic Periodontitis with Diabetic Mellitus patients which supports the etiological role of free radical injury .
Keywords
Chronic Periodontitis; Diabetes Mellitus; ROM; Plasma; Saliva; GCF
Download this article as:| Copy the following to cite this article: Umasudhakar, Ramakrishnan T, Rekha, Meena B, Kannadasan K, Amizhselvan T, Shankarram V. Prevalence of Reactive Oxygen Metabolites Levels in Chronic Periodontitis with Diabetic Mellitus and Chronic Periodontitis Patients in GCF, Plasma and Saliva –A Biochemical Study. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Umasudhakar, Ramakrishnan T, Rekha, Meena B, Kannadasan K, Amizhselvan T, Shankarram V. Prevalence of Reactive Oxygen Metabolites Levels in Chronic Periodontitis with Diabetic Mellitus and Chronic Periodontitis Patients in GCF, Plasma and Saliva –A Biochemical Study. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3745 |
Introduction
Periodontitis is a term used to describe an inflammatory process, initiated by the plaque biofilm, that lead to loss of periodontal attachment to the root surface and adjacent alveolar bone, and which ultimately results in tooth loss1.The gram negative anaerobes are the primary etiologic agents2 but majority of breakdown occurs as a result of inappropriate host response .PMNS are the primary mediators of host response against pathogens and they generate increased levels of Reactive Oxygen Species(ROS)3.
ROS is a term collectively describing the hydroxyl(OH-), hydroperoxyl (HOO-), nitric oxide( NO-), alkoxyl (Ro–), singlet oxygen(1O2), hypochlorous acid (HOCl) and hydrogen peroxide (H2O2)4. The changes in the host response as a consequence of various diseases can affect the severity and prognosis of periodontitis .One such disease is Diabetes Mellitus (DM),which encompasses a group of metabolic disorders characterized by hyperglycemia secondary to defects in insulin secretion, action (or) both. It has been reported that periodontitis is one of the first clinical manifestation of diabetes5 and is recognised as a common complication in diabetic patients particularly in poorly controlled diabetes 6. Diabetes Mellitus may contribute to the destructive aspects of the host response that leads to periodontal disease and other inflammatory conditions through the generation of reactive oxygen species (ROS)(7-10).Overproduction of ROS is also thought to contribute to the severity of the disease,including vascular disease(11-12).ROS are unstable , with a short half life , and thus are difficult to detect. Several markers of lipid peroxidation have been studied to monitor the ROS production. Hydroperoxides are one of the most studied marker of ROS.
In the present study ROM level is compared among subjects with Chronic periodontitis(CP) and Chronic periodontitis with Diabetes mellitus(CP-DM).
Materials and Methods
The study population consisted of 40 subjects belonging to both sexes and all subjects were randomly selected from the outpatient clinic of the Department of Periodontics, Thaimoogambigai Dental college and Hospital, Chennai. Subjects were divided into two groups of 20 subjects each as with Group I- Chronic Periodontitis (CP)and Group II- Chronic Periodontitis with Diabetes Mellitus (CP-DM). The participants were informed about the study and their consent was obtained .The study protocol was approved by the ethical committee of Dr. M.G.R University,Chennai , India, in accordance with the Helsinki Declaration of 1975,as revised in 2000.
Selection criteria
Inclusion criteria
Chronic Periodontitis
- Visible plaque
- Bleeding on probing
- At least four teeth with one or more sites exhibiting probing depth ≥ 4mm.
- Clinical attachment level ≥4mm.
Diabetic Mellitus with Chronic Periodontitis
- Known diabetic
- Fasting blood glucose level should be ≥120 mg/dl
- Bleeding on probing
- At least four teeth with one or more sites exhibiting probing depth ≥ 4mm.
- Clinical attachment level ≥4mm.
Exclusion criteria
- ≤ 15 teeth
- Current or previous smokers
- Pregnant or lactating women
- Presence of any systemic condition apart from Diabetes Mellitus for the DM-CP group.
- Subjects under antioxidants (vitamin C, vitamin E and coenzyme Q),antibiotics, or anti-inflammatory drugs in the past 3 months.
Collection of GCF
Detailed case history, clinical examination and supragingival scaling were done one day before the collection of GCF. On the subsequent day after drying the area with a blast of air, supragingival plaque was removed without touching the marginal gingiva and the GCF was collected. A standardized volume of 1µl was collected from each site with an extracrevicular approach, using volumetric capillary pipettes that were caliberated from 1-5µl. The collected GCF was transferred immediately to ependorff tubes and stored at -70◦c until the time of assay.
Collection of Plasma
Blood (3 ml) was drawn from the antecubital vein, under aseptic precautions and collected in a colour coded herparinised test tube (green colour) and centrifuged immediately at 3000 xg for 5 minutes. If not immediately assayed plasma aliquots were stored at -80ºc until analysis.
Collection of Saliva: Draining / Spitting method
The subject is asked to accumulate saliva in the floor of mouth and then spit into a preweighed or graduated test tube.
Laboratory method for detection of ROM
The d-ROMs test, developed by world-renowned Italian biochemist is a photometric test for measurement of the concentration of hydroperoxides (ROOH) in biological samples. The presence of ROOH in cells indicates oxidative attack of ROS on various organic substrates such as carbohydrates, lipids, amino acids, proteins, or nucleotides.
Test principle
The d-ROMs test uses the principle of Fenton’s reaction: by mixing a biological sample with an acidic buffer (Reagent R1), the newly-created transition metal ion (iron or copper) catalyzes the breakdown of hydroperoxide, generating new radical species such as hydroxyperoxyl (ROO+) and alkoxyl (RO+). By adding a chromogen (N, N-diethyl-paraphenylendiamine, Reagent R2) having the ability to donate an electron and change color when oxidized by free radicals, and using photometric reading available with the FRAS 4 dedicated analytical equipment, it becomes possible to quantify the level of hydroperoxides available in the sample.
Statistical Analysis
One way Anova is used to test the significance of the values in Chronic periodontitis(CP) and Chronic periodontitis with Diabetes Mellitus patients(CP-DM) .
Results
The mean value of ROM in GCF is 531.590, in plasma it is 565.628 and in saliva is 580.471 in Chronic periodontitis patients. The p value is 0.004.
In CP-DM patients , the mean value of ROM in GCF is 594.275, in plasma it is 630.165 and in saliva it is 635.555 . The p value is 0.007. This is depicted in Table 1.
On comparing the ROM values between groups there was a significant value in GCF(p value is 0.001).The ROM values between Chronic periodontitis(CP) and Chronic periodontitis with Diabetes Mellitus ( CP -DM) in plasma p value is <0.001 which is highly significant . On comparing the ROM values between CP and CP -DM in saliva ,the p value is <0.001 which is highly significant.This is depicted in Table 2.
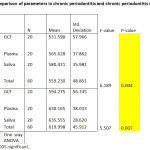 |
Table 1: Comparison of parameters in chronic periodontitis and chronic periodontitis with diabetes . |
 |
Table 2: Comparison of ROM values between groups in GCF, Plasma and Saliva. |
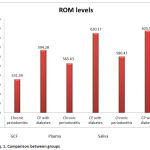 |
Figure 1: Comparison between groups |
Discussion
In this study the results demonstrated a significantly lower ROM levels in Chronic periodontitis (CP)when compared with Chronic periodontitis with diabetes mellitus (CP-DM). This was in accordance to the study by Brock et al which showed a reduction in systemic and local antioxidant (AO) defense in periodontitis13.
This is one of the first study which evaluates the ROM levels in GCF , plasma and saliva in CP-DM and CP patients . In the present study there was a significant difference in GCF ROM level between CP-DM and CP (p value 0.001). This may be attributable to the excessive oxygen radical production from antioxidation of glucose 14, glycated proteins and glycation of oxidative enzymes which limit their capacity to detoxify oxygen radicals15.
In the present study there was a highly significant difference between the ROM levels in plasma in CP-DM and CP patients . This was similar to the study done by Alliny et al 201216.The disturbed status in antioxidant mechanism may be explained on the basis of increased ROS production by phagocyte cells in periodontitis. The strongest evidence to implicate ROS in the pathological destruction of the connective tissue during periodontal disease arises in considering PMNS infiltration as a key event of the host response against microbial invasion. These infiltration in numbers is likely to lead to an increase in the ROS levels leading to oxidative stress17.
There was a highly significant difference between the salivary ROM levels in CP-DM and CP patients (p<0.001).Changes in the glucose level have been demonstrated to influence the Cu, Zn-SOD activity .Hyperglycemia induce superoxide formation and overproduction of superoxide also appears to be the central mechanism underlying all the major molecular mechanisms implicated in glucose –mediated vascular damage . This could be the reason for a variety of hyperglycemia-induced phenotypes in the target cells of diabetic complications18 .Decreased SOD( superoxide dismutase) activity and increased number of leukocytes could be attributable to an increase in ROS level, resulting in tissue breakdown. When compared to CP patients, CP-DM patients had increased ROM level. A decrease in the activities of antioxidant enzyme systems in Diabetes Mellitus is linked to the progressive glycation of enzymatic proteins. Approximately 50% of SOD in erythrocytes of patients with Diabetes Mellitus is glycated ,resulting in low activity19.
In our study ROM levels was compared in GCF, plasma and saliva .There were significant results obtained .Sampling of GCF is time consuming and might only reflect on gingival inflammation at the specific site sampled .Where as there is abundance of saliva and sampling is much easier, less costly, and could be better tolerated by the patient and analysis may provide an overall assessment of disease status as opposed to site specific GCF analysis20.In our study comparable results were obtained in saliva and plasma.
This study has its own limitation ,in that its non-longitudinal design made it difficult to evaluate the effect of periodontal treatment and Diabetes Mellitus control on ROM levels.
Conclusion
This study gives convincing support in favour of the etiological role of free radical injury in Chronic periodontitis with Diabetes Mellitus. The compensatory mechanism of antioxidants is collapsed because of excessive production of free radicals during periodontitis and is not able to cope with the increased production of free radicals attributable to Diabetes Mellitus and thereby could worsen the situation.
Bibliography
- Genco R, Kornman K, Williams R, et al. Consensus report. Periodontal diseases: Pathogenesis and microbial factors. Ann Periodontol 1996;1:926-932.
- Haffajee AD, Socransky SS. Microbial etiological agentsof destructive periodontal diseases. Periodontol 2000,1994;5:78-111.
- Moseley R, Waddington RJ, Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes.Biochim Biophys Acta 1997;1362:221-231.
- Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med 1999;10:458-476.
- Lamster IB, Lalla E, Borgnakke WS, Taylor GW. 2008. The relationship between oral health and diabetes mellitus. J Am Dent Assoc 139(Suppl):19S–24S.
- Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993;16:329-334.
- Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol 1997;24:287–296 .
- Panjamurthy K, Manoharan S, Ramachandran CR..Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol Biol Lett .200510; 255–264 .
- Akalin FA, Baltacioðlu E, Alver A, Karabulut E.Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis.J Clin Periodontol 2007;34:558–565.
- Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation. A possible role in the induction and progression of chronic periodontitis.J Periodontal Res.2005;40:378–384.
- Halliwell B.Oral inflammation and reactive species: a missed opportunity?Oral Dis 2000.;6:136–137.
- Velázquez E, Winocour PH, Kesteven P, Alberti KG, Laker MF. Relation of lipid peroxides to macrovascular disease in type 2 diabetes.Diabet Med1991.;8:752–758.
- Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health.J Clin Periodontol. 2004;31:515–521.
- Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 1993;49:642-652.
- Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: Which role for oxidative stress? Metabolism 1995;44:363-368.
- Alliny S. Bastos, Dana T. Graves, Ana Paula de Melo Loureiro, Carlos Rossa Júnior. Lipid Peroxidation Is Associated with the Severity of Periodontal Disease and Local Inflammatory Markers in Patients with Type 2 Diabetes. J Clin Endocrinol Metab. 2012 Aug; 97(8): E1353–E1362.
- Miyasaki KT, Wilson ME, Brunetti AJ, Genco RJ. Oxidative and non-oxidative killing of Actinobacillusactinomycetemcomitans by human neutrophils. Infect Immun. 1986;53:154–160.
- Sechi LA, Ceriello A, Griffin CA, Catena C, Amstad P, Schambelan M, et al. Renal antioxidant enzyme mRNA levels are increased in rats with experimental diabetes mellitus. Diabetologia. 1997;40:23–29.
- Arai K, Maguchi S, Fujii S, Ishibashi H, Oikawa K, Taniguchi N. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J Biol Chem 1987;262:16969-16972.
- Miller CS, King CP Jr., Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease:A cross-sectional study. J Am Dent Assoc 2006;137:322-329.







