Amit K. Dutta1, N. Ganesh1, C. S. Senthilkumar1 and N. K. Sah2
1Department of Research, Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal.
2School of Biotechnology, Rajiv Gandhi Technical University, Bhopal.
Abstract
Chronic Myeloid leukemia (CML) is a blood cancer of myeloid progenitors initiated with a triphasic absolutely curable with a targeted gene drug Imatinib mesylate. This study was conducted to reveal the effectiveness of Imatinib mesylate in 20 CML patients subjected with a follow – up of 9 months to evaluate their remission of the disease status. Complete hematological and Cytogenetic analysis was performed to find out the response of Imatinib mesylate against CML in every follow – up. Majority of the CML patients had showed a positive response in decreased level of abnormal cell proliferation and loss of Philadelphia positive chromosome(Ph+ve) in their profile. Our research evidenced a potential activity of Imatinib mesylate as a targeted gene therapy drug against CML.
Keywords
Chronic Myeloid Leukemia; Imatinib Induction; Long term follow – up studies
Download this article as:| Copy the following to cite this article: Dutta A. K , Ganesh N , Sah N. K ,Senthilkumar C. S. Longterm Follow – up of cml Cases with Imatinib Induction: A Hematological and Cytogenetic based Report. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Dutta A. K , Ganesh N , Sah N. K ,Senthilkumar C. S. Longterm Follow – up of cml Cases with Imatinib Induction: A Hematological and Cytogenetic based Report. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=365 |
Introduction
Leukemia is a group of disease characterized by abnormal proliferation of bone marrow and leukocytes. Leukemia can be classified according to their cell type namely lymphocytic, myelocytic and also as per the infection phase such as chronic or acute.
Chronic Myeloid leukemia (CML) is a commonly diagnosed blood cancer in adults. Basically, the course of the disease is triphasic starting with a chronic phase lasting 3 to 6 years followed by transformation to accelerated phase and finally with the short duration blast crisis phase1-5. Major cause of CML depends on the basis of molecular mechanism involved in the translocation of BCR and ABL genes leads to the formation of BCR – ABL fusion gene. As a resultant, there will be a reciprocal translocation of chromosome 9 and 22 to form t(9;22) Philadelphia chromosome5-10. The molecular combination of two genes BCR and ABL forms an aberrant BCR – ABL gene in chromosome 22. The chimeric BCR – ABL protein results in elevated tyrosine kinase activity.
CML can be curable with allogenic stem cell transplantation. Treatment with interferon α can induce a complete cytogenetic response, but it is associated with serious side effects. Patients those interferon therapy fails are usually treated with hydroxyurea or busulfan11. Imatinib mesylate (Gleevec, Novartis, Switzerland) formerly called as STI – 571and CGP 57148B is one of the recent drug evolved a great attention in treatment of CML. It exhibits a little host toxicity and inhibits the phosphorylation of stem cell factor receptor and platelet derived growth factor (PDGF)12. The major purpose of our work is to study effectiveness of Imatinib in CML patients with a follow – up to prove the hematological and cytogenetic response. Control of abnormal cell proliferation that attains a normal range in hematological profile and loss of Philadelphia chromosome in their cytogenetic profile concludes the overall remission of the disease status.
Patients and Methods
The follow – up study was conducted in 20 CML patients of age group more than 18 years those enrolled in JNCHRC for their treatment whose previous interferon therapy had failed.
Pedigree analysis and Hematological Profile
Prior to the Imatinib mesylate therapy, the patients were subjected to pedigree analysis and their complete blood profile, liver functioning, serum creatinine and electrolytes were noted. On every month follow – up hematological picture was performed to evaluate Complete Hematological response (CHR) to Imatinib mesylate
Cytogenetic analysis
For cytogenetic analysis both 1 ml peripheral blood and 0.5ml bone marrow samples were collected in heparinized tubes in every follow – up visit and lymphocytes were cultured in RPMI – 1640 media (Gibco) with 300µl Phytohaemagglutinin (Gibco). Incubated the cultures in CO2 incubator at 37°C for 72 hours. After 70 hours, 50µl of Colchicine (Gibco) was added to the culture and incubated again. On the completion of 71 hrs, the cells were harvested with 0.57% KCl (Qualigens) and cell washed with cornoy’s fixative repeatedly for 3 – 4 times. Slides were prepared using air drop method and kept in aging at 60°C. Then the slides were subjected to GTG banding. Around 20 good metaphase with Philadelphia chromosome was examined and photographed under image analyzer (Motic, Japan).
Results and Discussion
Our study revealed some of the classical findings in CML patients underwent Imatinib therapy. A condition such as mild anemia, leukocytosis with a characteristic left shift, Basophilia and frequent thrombocytosis with large hypo granular platelets was significantly noted prior to the initiation of therapy. At the time of their 3rd month blood and bone marrow evaluation after therapy, out of 20 patients 17 had a documented CHR included a white blood cell count that was below 10 × 109/L and a platelet count below 450 × 109/L. The remaining patient had elevated WBC and platelet counts in 3, 6, and 9 month evaluations, with documented CHR only in 12 -15 months. 3 patients had a slight variation in their CHR. Previous research reported in Imatinib mesylate also supported our study regarding hematologic response that occurs typically within 4 weeks after initiation of Imatinib mesylate treatment with CHR in 6 weeks 13.
On treating with Imatinib mesylate, the WBC count decreased from 31 × 109/L pretreatment to a mean of 6.4 × 109/L in 3 months. Circulating myeloid precursors disappeared in all patients within 3 to 6 months. Platelet count also decreased from 438 × 109/L pretreatment to a mean of 285 × 109/L in 3 months. Decreasing in platelet count indicated the disappearance of large, atypical platelets which is a characteristic of CML. Absolute basophilic count decreased from 1.0 × 109/L pretreatment to a mean of 0.3 × 109/L in 3 months. Mild anemia was an occasional finding in CML patients prior to the treatment with Imatinib. Most of the patients experienced a mild decrease in hemoglobin levels after treating with Imatinib mesylate. Hemoglobin level also significantly decreased from 130 g/L to a mean of 123 g/L in 3 months. In 15 months, 13 out of 20 patients had mildly decreased hemoglobin compared with pretreatment values and 7 patients had slightly increased hemoglobin.
Cytogenetic responses of 20 CML patients had shown extremely variable after the Imatinib mesylate therapy. In over all 20 CML patients, there is no remission in 3 months of therapy. The complete remission and cytogenetic responses such as Philadelphia negative (Ph-ve) was observed in 12 patients in 6 months after starting Imatinib mesylate. 5 patients had partial cytogenetic responses (Ph-ve) in 9 months and rest 3 of the patients had still incomplete remission. Apart from this whole abnormality of t(9;22) (Fig – 1– A, B), Marker Chromosome (Fig – 2), Fragile 2nd (Fig – 3) was also observed in some CML Patients.
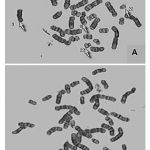 |
Figure 1:(A, B) Philadelphia chromosome t(9:22) of CML Patients. |
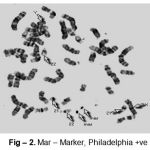 |
Figure 2: Mar–Marker, Philadelphia +ve |
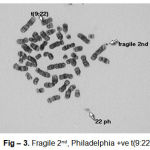 |
Figure 3: Fragile 2nd, Philadelphia +ve t(9:22) |
As compare to our knowledge, the routine hematological and cytogenetic follow – up after treating with Imatinib mesylate will help out in diagnosis of the various phase as well as recovery of the patients acquired with Chronic Myeloid Leukemia.
Our research study, proven the novel finding of Imatinib mesylate and its effectiveness in 20 CML cases. Imatinib mesylate, the first molecularly targeted therapy for CML results in a rapid and dramatic resolution of the Peripheral blood abnormalities associated with CML. CML patients who received interferon α-resistant and hydroxyurea also came with a good response to Imatinib mesylate resulted in a rapid and complete bone marrow morphological remission. The myeloid hyperplasia associated with CML typically resolves completely in 3 months, but atypical megakaryocytic hyperplasia persists up to 6 months in some patients. Subsequently, these CML patients had a quick response and remission after the follow – up interval of Imatinib mesylate treatment. Although, our research data is sufficient to predict the effectiveness Imatinib mesylate against CML, We suggest to extent the follow – up study in wide group of CML patients to know the factual mechanism of Imatinib mesylate as a potential targeted gene therapy against CML.
Acknowledgement
We thank our honorable chairman Mr. Madan Mohan Joshi, Director Dr. K. V. Pandya, Ex-Director Dr. B. Sanyal for providing research facilities. We also acknowledge Dr. T. P. Sahoo and Dr. Ritu Singhal and entire team of JNCHRC for their kind support.
References
- Fedrl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 131: 207- 19 (1999).
- Kantarjian HM, Deisseroth A, Kurzock R, Estrow Z, Talpaz M. Chronic myelogenous leukemia; a concise update. Blood, 82: 691- 703 (1993).
- Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukemia seen at a referral centre over a 16- year period. Br J Haematol, 96: 111- 6(1997).
- Speck B, Bortin MM, Champlin R et al. Allogeneic bone- marrow transplantation for chronic myelogenous leukemia. Lancet, 1: 665 – 8 (1984).
- Sokal JE, Baccarani M, Russo D, Tura S. Staging and prognosis in chronic myelogenous leukemia. Semin Hematol, 25: 49 – 61 (1988).
- Sawyers CL. Chronic Myeloid leukemia. N Engl J Med, 340: 130 – 40(1999).
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature, 243: 290- 3 (1973).
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science, 132: 1497 (1960).
- Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale PK, Groffen J. Acute leukemia in Bcr-Abl transgenic mice. Nature, 344: 251-3 (1990).
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science, 247: 824- 30 (1990).
- Silver RT, Woolf SH, Hehlmann R, et al. An evidence- based analysis of the Effect of busulfan, hydroxyurea, interferon and allogenic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood, 94: 1517- 36 (1999).
- Druker BJ, Sawyers CL, Kantarjian H et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 344: 1038- 42 (2001).
- Druker BJ, Talpaz M, Resta D, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 344:1031-1037 (2001).







