Arshi Naqvi* , Mohd. Shahnawaaz , Arikatla V. Rao and Daya S. Seth
School of Chemical Sciences, Chemistry Department, St. John’s College, Agra
Abstract
Nitrogen containing heterocycles are frequently found in privileged pharmacophores. Pyrazolin-5-ones are important nitrogen-containing five-membered heterocyclic compounds and have been found to be associated with a broad spectrum of bioactivities. The present work is directed towards the synthesis of some substituted-3-methyl-2-pyrazolin-5-ones. The synthesis of title compounds was achieved by the reaction of substituted ²-keto ester with substituted thiosemicarbazides and the synthesized compounds were screened for their antibacterial activity.
Keywords
Ethyl-2-substituted phenyl hydrazono-oxobutyrate; Substituted thiosemicarbazides; Pyrazolin-5-one
Download this article as:| Copy the following to cite this article: Naqvi A , Shahnawaaz M , Rao A. V, Seth D. S. Synthesis and Antibacterial Evaluation of Substituted 3-methyl-2-pyrazolin-5-ones. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Naqvi A , Shahnawaaz M , Rao A. V, Seth D. S. Synthesis and Antibacterial Evaluation of Substituted 3-methyl-2-pyrazolin-5-ones. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=340 |
Introduction
Pyrazolone moiety (a five-membered lactam ring containing two nitrogens and ketone in the same molecule or alternatively a derivative of pyrazole possessing an additional carbonyl/hydroxy group) has been the focus of medicinal chemists for over last 100 years because of the outstanding pharmacological properties shown by several of its derivatives1,2 e.g. ampyrone, metamizole etc. The pyrazolone ring is the basis of agents with various biological activities including antihyperglycemic properties1, anti-tumor necrosis factor activity3-4, non-steroidal anti-inflammatory drugs (NSAIDs)5 ,inhibition of human telomerase6 and antibacterial activity7. Also, substituted 2-pyrazolin-5-ones play an important role as substructures of numerous pharmaceuticals, agrochemicals, dyes, pigments, as well as chelating agents and thus attract remarkable attention8-10. The mentioned properties prompted us to synthesize substituted-3-methyl-2-pyrazolin-5-ones.
Experimental
Material
All chemicals used in the synthesis were of analytical grade. Melting points were determined in open capillary tubes and are uncorrected. The purities of the compounds were checked on silica-gel-coated Al plates (Merck). IR spectra were recorded in KBr on a Perkin Elmer Spectrum RX-1 FT-IR spectrophotometer. 1H-NMR spectra was measured on Advance Bruker DRX-300. Elemental analysis was performed on Elementor Vario EL III.
Synthesis of Ethyl-2-substituted phenyl hydrazono-oxobutyrate(1a-c) 11
Substituted aniline (o-chloro, p-chloro and p-nitro)(0.01mole) was dissolved in a mixture of concentrated HCl (8 ml) and water (6 ml) and cooled to 0oC in an ice bath. To it a cold aqueous solution of sodium nitrate (0.03 mole) was added. The diazonium salt solution was added dropwise into a cooled solution of ethylacetoacetate (0.01 mole) and sodium acetate (0.12 mole) in ethanol (50 ml). The resulting solid was washed with water and recrystallized with absolute ethanol.
Synthesis of substituted thiosemicarbazides(2a-e) 12
To a solution of substituted aniline (p-methyl, o-methoxy, p-methoxy, 3,4-dimethyl and o-chloro) (0.01mole) in ammonia (20ml) and water (5ml), CS2 (7.5 ml) and ethanol (20ml) was added and stirred vigorously for 1 hour. Solution of Sodium carbonate(5.3 gm) and mono chloro acetic acid (9.5gm) in water (40ml) was added followed by hydrazine hydrate (6ml) and refluxed for 30-45 mins on steam bath. The resulting solid obtained on cooling was recrystallized with absolute ethanol.
General method for synthesis of substituted-3-methyl-2-pyrazolin-5-one (1-15)
To 1a-e (0.01 mole), ethanol (20ml) and 2a-e (0.01 mole) was added and refluxed for 1-2 hrs in presence of 2-4 drops of glacial acetic acid. The resulting solid obtained was cooled, filtered and was recrystallized with hot absolute ethanol.
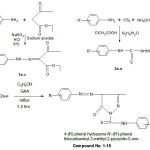 |
Scheme 1 |
Table 1: Physical and analytical data of compounds
| SI. No | R | R’ | Mol. Formula | Color | M.P
(oC) |
%Yield | % N
Found (Calc.) |
% S
Found (Calc.) |
| 1. | 2-Cl | 4-CH3 | C18H16ON5SCl | Orange | 195 | 80.31 | 18.18
(18.15) |
8.41
(8.30) |
| 2. | 2-Cl | 2-OCH3 | C18H16O2N5SCl | Yellow | 166 | 62.32 | 17.49
(17.41) |
8.07
(7.96) |
| 3. | 2-Cl | 4-OCH3 | C18H16O2N5SCl | Orange | 178 | 64.80 | 17.45
(17.41) |
8.05
(7.96) |
| 4. | 2-Cl | 3,4-di CH3 | C19H18ON5SCl | Orange | 165 | 60.00 | 17.56
(17.52) |
8.12
(8.00) |
| 5. | 2-Cl | 2-Cl | C17H13ON5SCl2 | Yellow | 170 | 56.43 | 17.27
(17.24) |
7.94
(7.88) |
| 6. | 4-Cl | 4-CH3 | C18H16ON5SCl | Orange | 170 | 73.03
|
18.21
(18.15) |
8.38
(8.30) |
| 7. | 4-Cl | 2-OCH3 | C18H16O2N5SCl | Orange | 165 | 70.35 | 17.51
(17.41) |
8.03
(7.96) |
| 8. | 4-Cl | 4-OCH3 | C18H16O2N5SCl | Orange | 164 | 68.36 | 17.49
(17.41) |
8.00
(7.96) |
| 9. | 4-Cl | 3,4-di CH3 | C19H18ON5SCl | Yellow | 169 | 65.79 | 17.61
(17.52) |
8.08
(8.00) |
| 10. | 4-Cl | 2-Cl | C17H13ON5SCl2 | Orange | 155 | 61.83 | 17.30
(17.24) |
7.92
(7.88) |
| 11. | 4-NO2 | 4-CH3 | C18H16O3N6S | Orangish
Yellow |
191 | 72.33 | 21.30
(21.21) |
8.14
(8.08) |
| 12. | 4-NO2 | 2-OCH3 | C18H16O4N6S | DarkYellow | 194 | 53.29 | 20.37
(20.31) |
7.72
(7.76) |
| 13. | 4-NO2 | 4-OCH3 | C18H16O4N6S | Orange | 168 | 50.36 | 20.39
(20.31) |
7.73
(7.76) |
| 14. | 4-NO2 | 3,4-di CH3 | C19H18O3N6S | Brick
Red |
170 | 71.04 | 20.47
(20.42) |
7.85
(7.80) |
| 15. | 4-NO2 | 2-Cl | C17H13O3N6SCl | Golden
Yellow |
190 | 61.83 | 20.13
(20.16) |
7.63
(7.68) |
Table 2: Characterization data of compounds
| Compound No. | IR
(ν in cm-1) |
1H NMR
(δ in ppm) |
| 1 | 1110 (C=S), 1571 (-N=C-, pyrazolone ring), 1674 (>C=O) | 1.22 (s, 3H, CH3), 2.34 (s, 3H, CH3), 3.38 (s, 1H, pyrazolone ring), 4.60 (s, 1H, NH), 6.35-7.86 (m, 8H, Ar-H). |
| 6 | 1108 (C=S), 1574 (-N=C-, pyrazolone ring), 1673 (>C=O) | 1.25 (s, 3H, CH3), 2.35 (s, 3H, CH3) , 3.36 (s, 1H, pyrazolone ring), 4.65 (s, 1H, NH), 6.42-7.70 (m, 8H, Ar-H) |
| 11 | 1115 (C=S), 1560 (-N=C-, pyrazolone ring), 1686 (>C=O) | 1.30 (s, 3H, CH3), 2.37 (s, 3H, CH3), 3.40 (s, 1H, pyrazolone ring), 4.73 (s, 1H, NH), 6.55-8.65 (m, 8H, Ar-H), |
Antibacterial screenings
Filter paper disc technique using Hi-Media agar medium is employed to study the antibacterial activity of 1-15 against Staphylococcus aureus and Escherichia coli. The concentration of test compounds is 1,000 µg/ml. After 48 hr incubation at 37 oC, zone of inhibition produced by each compound is measured in mm as shown in Table 3. Streptomycin is used as the reference drug and Dimethyl formamide as a control.
All tested compounds showed slight to moderate antibacterial activity.
Key to symbols: Resistance = R; slightly active = + (inhibition zone 6-9mm); moderately active = + + (inhibition zone 9-12 mm); highly active = + + + (inhibition zone> 12 mm).
Table 3: Antibacterial activity of the compounds I-XII
| Compound No. | E. coli | S. aureus |
| 1 | ++ | R |
| 2 | + | + |
| 3 | + | R |
| 4 | + + | + + |
| 5 | + | R |
| 6 | ++ | + + |
| 7 | + | R |
| 8 | + | R |
| 9 | + + | + |
| 10 | + + | R |
| 11 | + | + |
| 12 | + | R |
| 13 | ++ | + |
| 14 | ++ | ++ |
| 15 | + | R |
| Streptomycin | + + + | + + + |
Acknowledgements
We are thankful to Central Drug Research Institute (CDRI), Lucknow for spectral and microanalysis and Dr. B.M. Agarwal, S.N.Medical College, Agra for antibacterial screenings.
References
- Kees, K. L.; Fitzgerald, Jr. J. J.; Steiner, K. E.; Mattes, J. F.; Mihan, B.; Tosi, T.; Mondoro, D.; McCalebr, M. L.; J. Med. Chem., 39, 3920 (1996)
- Pal, S.; Mareddy, J.; Devi, N.S.; J. Braz. Chem. Soc., 19, 6, 1207-1214 (2008).
- Clark, M. P.; Laughlin, S. K.; Laufersweile, M. J.; Bookland, R. G.; Brugel, T. A.; Golebiowski, A.; Sabat, M. P.; Townes, J. A.; VanRens, J. C.; Djung, J. F.; Natchus,
- G.; De, B.; Hsieh, L. C.; Xu, S. C.; Walter, R. L.; Mekel, M. J.; Heitmeyer, S. A.; Brown, K. K.; Juergens, K.; Taiwo, Y. O.; Janusz, M. J. J. Med. Chem., 47, 2724 (2004).
- Laufersweiler, M. J.; Brugel, T. A.; Clark, M. P.; Golebiowski, A.; Bookland, R. G.; Laughlin, S. K.; Sabat, M. P.; Townes, J. A.; VanRens, J. C.; De, B.; Hsieh, L. C.; Heitmeyer, S. A.; Juergens, K.; Brown, K. K.; Mekel, M. J.; Walter, R. L.; Janusz, J. Bioorg. Med. Chem. Lett., 14, 4267 (2004).
- Schillaci, D.; Maggio, B.; Raffa, D.; Daidone, G. Farmaco, 47, 127 (1992).
- Kakiuchi, Y.; Sasaki, N.; Satoh-Masuoka, M.; Murofushi, H.; Murakami-Murofushi, Biochem. Biophys. Res. Commun., 320, 1351 (2004).
- Soliman, R.; Habib, N. S.; Ashour, F. A.; el-Taiebi, M. Boll. Chim. Farm., 140, 140 (2001).
- J. Elguero, In ‘Comprehensive Heterocyclic Chemistry: Pyrazoles and their Benzo Derivatives’, Vol. 5; A. R. Katritzky and C. W. Rees, Eds., Pergamon Press, Oxford, 167–303(1984).
- Stanovnik, B.; Svete, J. Product class 1: Pyrazoles. Science of Synthesis, 12, 15–225 (2002).
- Eller, G.A.; Holzer, W.; Molbank, M464 (2006).
- Amir, M.; Hasan, S.M.; Wadood, A.; Orient. J. Chem.; 18(2), 351 (2002).
- Desai, N.C.;Parekh, B.R.; Thaker, K.A.; J. Ind. Chem. Soc. LXIV, 491-493 (1987); Jensen, K. A.; J. Prakt. Chem. 159, 189 (1964)







