R.K. Jangde, M. Singh and D. Singh*
University Institute of Pharmacy, Pt. RavishankarShukla University, Raipur, Chhattisgarh, India 492010.
DOI : https://dx.doi.org/10.13005/bpj/541
Abstract
Rutin is a polyphenolic naturally occurring compound potentially having a huge impact in human benefit which includes wound healing, cardiovascular, carcinoma therapeutic activities as well as have nutraceutical benefits. The objective of study was to establish a precise, accurate, sensitive and reliable method for estimation at low concentrations of rutin in biological fluids, using spectrofluorimetric method.A standard curve of rutin was prepared in methanol and serum in the concentration range 1-10 ng/ml. The method was validated in terms of linearity, accuracy and precision.The regression data for rutin in methanol and serum observed to have a good linear relationship with R2> 0.99 over the concentration range of 1-10 ng/ml. This method gave adequate linearity, precision, accuracy and recovery. It is also concluded that method is less time consumption as compared with HPLC, which requires lengthier chromatographic separation.
Keywords
Rutin; spectrofluorimetric; Accuracy and precision
Download this article as:| Copy the following to cite this article: Jangde R. K, Singh M, Singh D. Micro Calcifications Detection for Breast Cancer Diagnosis using Infrared Thermal Imaging Apparatus. Biomed Pharmacol J 2014;7(2) |
| Copy the following to cite this URL: Jangde R. K, Singh M, Singh D. Micro Calcifications Detection for Breast Cancer Diagnosis using Infrared Thermal Imaging Apparatus. Biomed Pharmacol J 2014;7(2). Available from: http://biomedpharmajournal.org/?p=3259 |
Introduction
Bioflavonoids are polyphenol organic compound derived from the plants. Reactive oxygen species play important role in inception of numerous diseases. Antioxidant substances have capacity to scavenge the free radical or reactive oxygen species present in body. Polyphenol component exhibited strongly antioxidant activity. It is documented that the polyphenol are used for the healing of various diseases namely cardiovascular, ulcers, cancer, hepatotoxic, arthritis, hypertension etc[1-3]. Rutin(5, 7, 3’, 4’, tetrahydroxyflavonol -3- rhamnoglucoside) is most abundant of the flavonoids, and consist of 3 rings and 4 hydroxyl groups (figure 1).Rutin occurs in food as an aglycone (attached to a sugar molecule). Rutin is a member of bioflavonoids also called vitamin P with antioxidant, anti-inflammatory, antiallergenic, antiviral, and anti-carcinogenic including anti-tumor, antidiarrhoeal, anti-mutagenic, myocardial protecting and immunomodulatory properties and has been demonstrated to scavengesuperoxide radicalswhich make them potential therapeutic agent. Rutin have aromatic essence for which it is also used in nutraceuticals, rutin along with other flavonoids are highly used for wound healing because of its antioxidant property [4-5]. The potent antioxidant property of rutin credits by scavenging oxygen radicals which terminates free radical chain reaction, conversion of superoxide to reactive hydroxyl radicals and inhibits lipid peroxidation preventing tissue damage which significantly improves wound healing [6-9]. The activity of rutin make them potential agent for treatment of various skin problems like cancer, eczema, sun burn. It is also used for treatment of diseases like hepatotoxicity, cardiovascular and carcinoma. Now a day’s rutin is key component of various cosmaceuticals especially for anti-aging and nutraceuticals[10].Various methods have been determined for estimation of rutin and its metabolite such as liquid chromatographic and high performance liquid chromatography (HPLC) techniques [11]. Recent established method involves infrared spectroscopy, mass spectroscopy, Nuclear magnetic spectroscopy to identify and quantitate rutin [12-13]. Kale and laddha has also been reported for determination of the total flavonoid content and quantitative estimation of rutinby RP-HPLC [14]. Pawar and Salunkhe similarly performed analysis of rutin by U.V. Spectrophotometry [15]. Luftmann et al performed analysis of rutin by mass spectrometry, 1H NMR spectrometry and HPTLC in rat urine. This process requires tedious processing and long time for analysis of sample [16]. Another limitation of existing method includes non suitability for estimation of rutin in biological samples, as the limit of detection is 50 ng/ml [17]. Apart from above, all the HPLC methods require a long run time for analysis which is based on the retention time of rutin in the mobile phase used. The main problem with rutin is its instability at neutral or basic pH and, also, it’s very poor absorption when taken orally or applied topically. Therefore, a precise, reliable and sensitive method is necessary to estimate rutin in biological fluids [18-20]. Sethiya et al. developed a spectrofluorimetric method for the estimation of scopoletin and mangiferin in different varieties of Shankhpushpi [21]. Till now no work has been performed for analysis of rutin by spectrofluorimetric method in goat serum. Present work has been designed for analysis of rutin in goat serum with the aim to develop a precise, accurate, sensitive and reliable method for determination of rutin in low concentrations, using spectrofluorimeter.
Materials
Rutin was purchased from Hi-media chemicals (Mumbai, India). Methanol purchased from Merck (Mumbai, India). HPLC grade solvents were purchased from Merck (Mumbai, India). All other materials and solvents used were of analytical grade.
Experimental Methods
The spectrofluorimetric study was carried out with a Shimadzu RF 5301 PC spectrofluorimetric, to determine the level of fluorescence of the phenolic compounds in a stationary state. The light source used was a xenon 150 W lamp with an optical system composed of two automatic mono chromators, one for excitation and the other for emission. The detection system was an R 450-01 photomultiplier which transformed the fluorescent radiation emitted by the rutin solution in the cell into an electrical signal [19].
Preliminary analysis and preparation of a standard curve in methanol
A preliminary analysis was carried out to determine the wavelength at which maximum intensity
exhibited by pure rutin. For this purpose, a 10 ng/ml sample of pure rutin was prepared in methanol. This was scanned spectrofluorimetrically to obtain the excitation and emission wavelengths. The λ max shown by rutin had an excitation at 340 nm and an emission at 350 nm.
A standard curve of rutin was prepared in concentration range of 1-10 ng/ml. First of all, a stock solution containing 100 µg/ml of rutin was prepared in methanol. This stock solution was further diluted to obtain required dilution containing 1 to 10 ng/ml rutin. These solutions were analyzed and a standard curve obtained by plotting the concentration of rutin versus the intensity of fluorescence.
Analytical method validation
Linearity
Standard solutions (1 ng/ml to10 ng/ml) were prepared in methanol and the intensity of fluorescence was recorded in the spectrofluorimeter. The standard curve was prepared by plotting the concentration as the abscissa versus the intensity of fluorescence as the ordinate. A linear dependence of intensity on concentration was observed over the entire concentration range tested.
Precision and accuracy
The precision of the method was checked using diluted methanolic solution of rutin with volumes of 10, 20, 50 and 100 ml. The solutions were analyzed in a spectrofluorimeter at (excitation and emission wavelengths) for rutin and the intensities were recorded. The entire procedure was repeated three times for each dilution and the reading were expressed as mean ± S.D. (n=3). Then, an approximately 1ng/ml solution of rutin was prepared by diluting the stock solution of rutin in methanol and analyzed in the spectrofluorimeter. The concentration of rutin in this solution was also calculated from the standard curve. Then 2, 4, 6, 8and 10 ng/ml of this standard rutin were solution. These sample were then analyzed to see whether the observed concentration corresponded to the theoretical concentration from the standard curve and the % recoveries were calculated(Table1)
Table 1: Validation of the spectrofluorimetric method (percentage recovery of rutin)
| S.No. | Volume of Standard rutin solution (ng/ml) [a] | Calculated amount of rutin in the standard solution (ng)[b] | Amount analyzed (ng) | Percentage recovery |
| 1 | 2 | 0.93 | 9.36±0.34 | 96.42±1.41 |
| 2 | 4 | 1.89 | 20.41±0.23 | 98.34±2.52 |
| 3 | 6 | 3.72 | 50.00±0.25 | 98.99±2.40 |
| 4 | 10 | 9.25 | 100.00±0.30 | 100.00±0.30 |
*As calculated from the standard curve. All values are Mean±S.D.(n=3)
Excitation(λmax:350nm) Emission(λmax: 490nm)
Estimation of rutin in biological fluid (serum)
Rutin was also estimated in goat serum, to explore the possibility of use of the spectrofluorimetric method for determination of the rutin concentration in biological fluids. For this purpose a standard curve of rutin was prepared in goat serum. In the present method we used buffer solution (PBS, pH 7.4) for dilution of the serum to maintain the acidic medium during the estimation process. First of all, goat blood was collected from slaughter home at Raipur market and serum was separated by centrifugation at 5000 rpm for 30 min. The isolated clear serum was diluted with buffer solution (PBS, pH 7.4) to 10%. Then, 10 mg rutin was weighed and dissolved in a minimum volume of methanol. Then, the volume was made up to 100 ml with diluted serum and this stock solution was used to prepare required dilutions containing 1ng/ml to 10ng/ml rutin. The samples were analyzed in the spectrofluorimeter against solvent blank (diluted serum). The wavelength and intensity of each sample was recorded and a standard curve was prepared using the concentration versus the intensity of fluorescence.
 |
Figure 1: Rutin
|
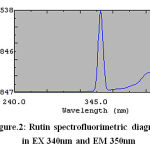 |
Figure 2: Rutinspectrofluorimetric diagram in EX 340nm and EM 350nm
|
Validation of the method in serum
Linearity
Standard solutions (1 ng/ml to10 ng/ml) were prepared in diluted serum and the intensity of fluorescence was recorded in the spectrofluorimeter.The standard curve was prepared by plotting the concentration as the abscissa versus the intensity of fluorescence as the ordinate. A linear dependence of intensity on concentration was observed over the entire concentration range tested.
Precision and accuracy
The precision of the method was checked by diluting the stock solution of rutin in serum to approximately 1ng/ml. Then, 2, 4, 6, 8 and 10 ng/ml of this solution were taken and analyzed in the spectrofluorimeter at excitation and emission wavelengths for rutin and the intensities were recorded. Rutin present in these solutions was calculated from the standard curve. The entire procedure was repeated three times for each dilution and the readings were expressed as Mean ±S.D. Then, 10 mg rutin was dissolved in a minimum volume of methanol and diluted to 10 ml with diluted serum. Then, 1 ml of this solution was further diluted to a concentration of approximately 1ng/ml. This solution was analyzed and diluted concentration of rutin was calculated. Then, 2,4,6,8 and 10 ml of this solution were added to the previous solutions. These samples were then analyzed to see whether the observed concentrations corresponded to the theoretical concentrations and the % recoveries were calculated (Table 2).
Table 2: Validation of the spectrofluorimetric method (percentage recovery of rutin in serum)
| S. No. | Volume of serum used (ng/ml) [a] | Calculated amount of rutin in the serum (ng) [a*] | Volume of standard solution added (ng/ml)[b] | Calculated amount of rutin in the standard solution (ng)[b*] | Total amount of rutin in mixture (ng)[a*+b*] | Amount analyzed(ng) | Percentage recovery |
| 1 | 1 | 0.93±0.04 | 1 | 0.930 | 1.86±0.04 | 1.864±0.06 | 98.50±0.215 |
| 2 | 2 | 2.14±0.36 | 2 | 2.520 | 4.66±0.36 | 4.321±0.13 | 99.421±0.05 |
| 3 | 5 | 4.45±0.15 | 5 | 4.470 | 8.92±0.15 | 9.025±0.09 | 100.15±0.25 |
| 4 | 10 | 9.52±0.50 | 10 | 9.852 | 19.372±0.50 | 19.375±0.22 | 100.05±0.68 |
*As calculated from the standard curve. All values are Mean±S.D.(n=3)
Excitation(λmax:350nm) Emission(λmax: 490nm)
Results and Discussion:
A standard curve for rutin was prepared at excitation and emission wavelengths of 340 nm and 350 nm, using a spectrofluorimeter. The plot of concentration versus intensity exhibited a linear relationship. The equation of the straight line for rutin was y = 0.0519x -0.0219. The percentage recovery of rutin was found to be in the range (99-100) % (Table 2). The rutincontent calculated from the standard curve was found to be 6.58 % w/w. The developed method was validated for linearity, reproducibility and accuracy. The linearity was found to be in the range 1-10 ng/ml. The correlation coefficient (r2) for rutin was 0.9972, indicating good linearity between the fluorescence intensity and concentration. Determining samples three timesallowed the precision of the method to be checked. The reproducibility and accuracy of the method was checked by carrying out recovery studies. Samples of known concentration were added in equal volume to different dilutions of the rutin analyzed spectrofluorimetrically to see whether the observed concentration obtained corresponded to the theoretical concentration obtained from the standard curve. The percentage recovery of rutin was found to be in the range (99-102) % (Table 1). The developed method is specific as the fluorescence of rutin is concentration dependent. Recovery studies in serum clearly indicate that interference from endogenous substances (if any) did not affect the intensity of fluorescence. Thus, the developed method is reliable for estimation of rutin in biological fluids. The method is very much simplified as compared to other, useful for very low rutin concentrations and is also less time consuming compared with HPLC, which requires a long chromatographic run time.
Conclusion
The developed spectrofluorimetric procedure is a quick and reliable method for the quantitative monitoring of rutin in very low concentrations in biological fluids, raw materials, processed powders and in herbal preparations of rutin.
Acknowledgement
The author, Dr. Deependra Singh is thankful to UGC-MRP41-748-2012, UGC-RA-70371/2012, DST-FIST and SAP. The authors are thankful to Director, University Institute of Pharmacy, Pt. Ravi Shankar Shukla University for providing facilities and guidance.
References
- Shahidi F. (ed.): Natural Antioxidants. Chemistry, Health Effects, and Applications, AOCS Press, Champaign, 1997;pp 210-15.
- Rice-Evans C.A., Packer L. (eds.):Flavonoids in Health and Diseases, Marcel Dekker, Inc., New York, 1998;pp325-30.
- Yumiko, N., Susumu, I., Yasuhide, T. Effects of Rutin and Rutin on Serum and Hepatic Lipid Concentrations, Fecal Steroid Excretion and Serum Antioxidant Properties. Journal of Health Science., 2000; 46(4): 229-40.
- Kale, M.K. S. and Laddha, K. S. Determination of total flavonoids content and quantification of rutin in Momordicatuberosa (Roxb)Cogn. fruits by RP-HPLC., Asian J. Trad. Med.,2012; 7(5):220-25.
- Ashraf, J., Siddique, J., Mirani, N., Rub, A. Protective effect of rutin against carbon tetrachloride-induced hepatotoxicity in mice. Int. J. D. Dev. & Res.,2012;4(2):352-7.
- Tiwari, P. and Patel, R. K. Development and Validation of HPTLC Method for Quantification of Quercetin and Rutin in Draksharishta. Asian J. Pharm. Sc. and Res.,2012; 2(1):7-18.
- Pawar,N. P. and Salunkhe, V. R. Development and Validation of HPTLC Method for Simultaneous Estimation of Rutin and Quercetin In Hydroalcoholic Extract of TriphalaChurna. Int. J. Pharm.Tech. Res.,2012; 4()4:1457-63.
- Ananth, P. H., Khan, S., Chandrasekhar, R., Ibrahim, M. Development of Validated HPTLC Method for Simultaneous Quantification of Rutin and Quercetin from Bark of AnogeissusLatifolia. Int. J. Pharm., 2012; 2(1): 33-38.
- Raote,A., Jangid, A., Tale, R., Vikas. Liquid Chromatography- Tandem Mass Spectrometric Method for Simultaneous Determination of Rutin and Quercetin from Leaves of ArtocarpusLakoochaRoxb. Int. J. Pharm. and Bio Sci., 2011; 2(1):848-53.
- Park,S.N., Lee,M.H.,Kim,S.J.,Yu,E.R. Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system., Biochem. andBiophys. Res. Com., 2013; 435:361-6.
- Morales, M.A., Lozaya,X. Calcium-antagonist effect of rutin on aortic smooth muscle. Planta. Med., 1994; 60: 313-17.
- Sajeeth, C.I., Manna, P.K., Manavalan, R., Jolly, C.I. Quantitative estimation of Gallic Acid, Rutin and Rutin in certain herbal plants by HPTLC method. Der ChemicaSinica., 2010; 1(2): 80-85.
- Boots, A.W., Haenen, G.R.,Bast, A. Health effects of rutin: from antioxidant to nutraceutical. Eur J Pharmacol., 2008; 58(5): 325–37.
- Wu, T.H., Yen, F.L., Lin, L.T., Sai, T.R., Lin, C.C., Cham, T.M. Preparation, physicochemical characterization, and antioxidant effects of rutin nanoparticles. Int J Pharm., 2008; 34(6): 160–68.
- Kim, Y.J., Bae, Y.C., Suh, K.T., Jung, J.S. Rutin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Bio.Chem.Pharmacol., 2006; 7(2):1268–78.
- Shaji, J., Iyer, S. Preparation, Optimization and In-Vivo Hepatoprotective Evaluation of Rutin Liposomes., Int. J.Curr. Pharm. Res., 2012; 4 (2): 24-32.
- Umathe, S.N., Dixit, P.V., Kumar, V., Bansod, K.U., Wanjari,M.M. Rutin pretreatment increases the bioavailability of pioglitazone in rats: involvement of CYP3A inhibition. BiochemPharmacol., 2008;75:1670–76.
- Chitkara, D., Kumar, S., Mittal, A., Chand, M., Kumar, N. Development of rutinnanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Del. and Transl. Res.,2012;2:112–23.
- Patil, S.J., Salunkhe, V.R., Alai, M.H. Estimation of Rutin in Ayurvedic Proprietary Medicine by UV-Spectrophotometry., Int. J. Pharm. Sci., 2012; 4(3): 645-47.
- Gupta, N.K., Nahata, A., Dixit, V.K. Development of a spectrofluorimetric method for the determination of curcumin. Asian J.Trad. Med., 2010; 5 (1):12-18.
- Sethiya, N.K., Nahata, A., Dixit, V.K. Simultaneous spectrofluorimetric determination of scopoletin and mangiferin in a methanolic extract of Canscora decussate Asian J.Trad. Med., 2008; 3 (6):224-29.







