Manuscript accepted on :
Published online on: 24-12-2015
Plagiarism Check: Yes
Titin Andri Wihastuti1, Djanggan Sargowo2, Mohammad Aris Widodo3, Teuku Heriansyah4, Setyowati Soeharto5 and Ahmad Izzuddin Ardi6
1Department of Biomedical, Medical Faculty, Brawijaya University, Malang, Indonesia.
2Department of Cardiology, Medical Faculty, Brawijaya University, Malang, Indonesia.
3Department of Pharmacology, Medical Faculty, Brawijaya University, Malang, Indonesia.
4Faculty of Medicine, Syiah Kuala University, Aceh, Indonesia.
5Department of Parasitology, Medical Faculty, Brawijaya University, Malang, Indonesia.
6Medical Faculty, Brawijaya University, Malang, Indonesia. Brawijaya University, Veteran street, Malang, East Java, 65145, Indonesia.
DOI : https://dx.doi.org/10.13005/bpj/506
Abstract
The current study evaluated the effect of subchronic toxicity of Ganoderma lucidum Polysaccharides peptide (PsP) to liver physiology and histopatology in rats. The 80 rats were randomly divided into four groups male and four groups female (10 rats per group): Three PsP treated group: 300, 600, 1200 mg/KgBW, and one normal control group. Aspartate aminotransferase (AST), Alanine aminotransferase (ALT) and Alkaline phosphate (ALP) levels of rats were measured, and histopathology changes: the average of cloudy degeneration, ballooning degenaration, the number of kupfer cell, inflamation cell and necrotizing cell were examinated, after 90 days of treatment with Ganoderma lucidum PsP, the ANOVA test show that AST, ALT and ALP levels of the PsP treated group was not significantly different than the control group, and the histopathology anatomy of the liver wasn’t show any different with the control group. These data suggest that Ganoderma lucidum PsP on all of the 3 doses was not cause any harm effect to liver enzymes neither to liver tissues.
Keywords
Subchronic;ganoderma lucidum PsP; liver; AST; ALT; ALP; histopathology anatomy
Download this article as:| Copy the following to cite this article: Wihastuti T. A, Sargowo D, Widodo M. A, Heriansyah T, Soeharto S, Ardi A. I. Subchronic Toxicity of Ganoderma Lucidum Polysaccharide Peptide (PsP) to Liver Physiology and HistopathologyImaging of Liver on Rattus Norvegicus Strain Wistar Rats. Biomed Pharmacol J 2014;7(2) |
| Copy the following to cite this URL: Wihastuti T. A, Sargowo D, Widodo M. A, Heriansyah T, Soeharto S, Ardi A. I. Subchronic Toxicity of Ganoderma Lucidum Polysaccharide Peptide (PsP) to Liver Physiology and HistopathologyImaging of Liver on Rattus Norvegicus Strain Wistar Rats. Biomed Pharmacol J 2014;7(2). Available from: http://biomedpharmajournal.org/?p=3022 |
Introduction
Ganoderma lucidum extract (Gl) (Leyss, an ex-Fr) have been widely use as an antidote to support health in China for thousands of years. Ganoderma lucidum extract and its components reportedly effective in the treatment hepatopathy chronic, hypertension, hyperglycemia and neoplasia in or pharmacology modern series in the last 30 years. However, the most interesting of benefits Gl is anti-tumor, which is shown related with the content polisakaridanya(Caoqz et al). Peptide Bonds Polysaccharide is one of the bioaktif that can be isolated and identified from fungus Ganoderma lucidum extract and some of the species fungi openely tell others (Xu Z., Chen X., Zhong Z., Chen L. , et all, 2011). Actively biology polysaccharide mainly comes from the branch (1→3)-β-D-glucans and may be pushed through the receptor component type 3 (CR3 receptor) that bound with polysaccharide (1→3)-β-D-glucans. Β-D-glucans component is a major component of Ganoderma mycelium components which is a minor from spores Ganoderma (Silva D. , 2003). To know safety of PSP treatment, so it needs to be tested the toxicity effects on organ and blood, one of which is to organs liver.
Liver are the organs that are very important in a profitable homeostasis body, on metabolism, biotransformation, synthesis, storage and immunology. These cells liver (hepatosit) have the ability to regeneration quickly. Thus until certain level, liver can maintain its function if there was small. In a more severe disorders, can cause malfunction that serious and fatal (DEPKES RI, 2007). Disorder or damage to liver can be seen from biomarker such as AST, ALT and ALP. If there is a damage in liver, then the biomarker will be increased. Furthermore from measurements biomarker liver, the liver condition can be seen also in histopathology to assess the inflammatory, degeneration, stockpiling intracellular, Steatosis (cause fatty liver), tissue necrosis and fibrosis.
PSP usage that has the benefit as modulation body’s immune system, antitumor, anti-atherosclerosis, anti-diabetes, anti-aging, and antioxidants that potent, and to test safety of using PSP for long periods of time, PSP subchronic test is done to the level AST, ALT, ALP and histopathology liver in Rattus norvegicus wistar strain rats.
Materials and Methods
Study group
The experimental animals were 80 Rattus norvegicus Wistar strain rats, 40 male and 40 female, aged 6 weeks with body weight of 100-150 g. Sample was obtained from CV. Gamma Scientific Biolab, Malang, Indonesia. Rats were divided into 4 groups (@10 rats): negative control and group with administration of PSP dose: 300, 600, 1200 mg/kgBW. PSP was gain from PT. Sahabat Lingkungan Hidup Surabaya. Administration of PSP was done with oral gavage once daily for 90 days. Measurement of parameters was conducted at the Laboratory Central, Saiful Anwar Hospital, Malang. Slicing and staining organ for histopathological observation was conducted at the Laboratory of Anatomic Pathology, Airlangga University, Surabaya, after we have obtained ethical clearance from the Health Research Ethics Committee by number: 400/112/K.3/302/2014.
Biochemical test
AST
AST levels were measured in rat plasma using photometry (Cobas c 311). This assay follows the recommendations oh the IFCC, but was optimized for performance and stability.AST in the sample catalyzes the transfer of an amino group between L-aspartate and 2-oxoglutarate to form oxaloacetate and L-glutamate. The oxaloacetate then reacts with NADH, in the presence of malate dehydrogenase (MDH), to form NAD+.
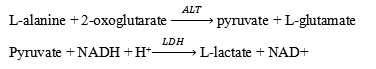
The rate of the NADH oxidation is directly proportional to the catalytic AST activity. It is determined by measuring the decrease in absorbance.
ALP
ALP levels were measured in rat plasma using Colorimetric assay in accordance with standardized method (Cobas c 311). In the presence of magnesium and zinc ions, p-nitrophenyl phosphate is cleaved by phosphatases into phosphate and p-nitrophenol.
![]()
The p-nitrophenol released is directly proportional to the catalytic ALP activity. It is determined by measuring the increase in absorbance.
Histopathology of Liver
Histopathological description of liver was observed by hematoxylin and eosin staining and microscope BX 53 (Olympus Corporation) at 200× magnification and quantitatively analyzed using OlyVIA software (version 2.8; Olympus Corporation). Histopathologi liver identification by examination of cloudy degeneration, ballooning degenaration, the number ofkupfer cell, inflamation celland necrosis cell.
Statistical analysis
This study used One-Way Analysis of Variance (ANOVA) to determine the effect of PSP on AST, ALT and ALP levels in Rattus norvegicus Wistar strain rats. Then Post Hoc test was performed to identify differences between groups. Statistical Software Product and Service Solutions (SPSS) software version 20 (IBM Corporation, 590 Madison Avenue, New York, USA) was utilized to obtain the data analysis.
Result
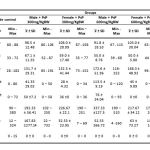 |
Table 1: Parameters measurement
|
Average AST levels in the negative control male group is 84 U/L, and the average AST level in the various treatment groups ranged between 91.8 – 98.5 U/L. ANOVA test with a 95% confidence level showed that administration of PSP had not a significant effect (p > 0.05) in the AST levels. While average AST levels in the negative control female group is 85.2 U/L, and the average AST level in the various treatment groups ranged between 88 – 108 U/L. ANOVA test with a 95% confidence level showed that administration of PSP had not a significant effect (p > 0.05) in the AST levels.
Average ALT levels in the negative control male group is 56.6 U/L, and the average ALT level in the various treatment groups ranged between 55 – 67.1 U/L. ANOVA test with a 95% confidence level showed that administration of PSP had not a significant effect (p > 0.05) in the ALT levels. While average ALT levels in the negative control female group is 52.6 U/L, and the average ALT level in the various treatment groups ranged between 50 – 59 U/L. ANOVA test with a 95% confidence level showed that administration of PSP had not a significant effect (p > 0.05) in the ALT levels.
Average ALP levels in the negative control male group is 139.9 U/L, and the average ALP level in the various treatment groups ranged between 117.2 – 172.6 U/L. ANOVA test with a 95% confidence level showed that administration of PSP had a significant effect (p < 0.05) in the ALP levels. Post Hoc Test with Duncan method showed that levels of ALP had not differed significantly between various treatment group and negative control group. While average ALP levels in the negative control female group is 86.7 U/L, and the average ALP level in the various treatment groups ranged between 132.3 – 168.3 U/L. ANOVA test with a 95% confidence level showed that administration of PSP had not a significant effect (p > 0.05) in the ALP levels.
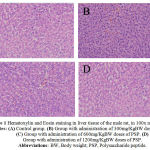 |
Figure 1: Hematoxylin and Eosin staining in liver tissue of the male rat, in 100x magnification. Notes: (A) Control group. (B) Group with administration of 300mg/KgBW doses of PSP. (C) Group with administration of 600mg/KgBW doses of PSP. (D) Group with administration of 1200mg/KgBW doses of PSP. Abbreviations:BW, Body weight; PSP, Polysaccharide peptide.
|
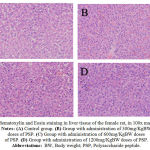 |
Figure 2: Hematoxylin and Eosin staining in liver tissue of the female rat, in 100x magnification. Notes: (A) Control group. (B) Group with administration of 300mg/KgBW doses of PSP. (C) Group with administration of 600mg/KgBW doses of PSP. (D) Group with administration of 1200mg/KgBW doses of PSP. Abbreviations:BW, Body weight; PSP, Polysaccharide peptide.
|
Average number of degeneration cloudy in the negative control male group is 69 cells, and the average number of degeneration cloudy in the various treatment groups ranged between 0 – 120 cells. While average number of degeneration cloudy in the negative control female group is 58 cells, and the average number of degeneration cloudy in the various treatment groups ranged between 0 – 92 cells.
Average number of degeneration ballooning in the negative control male group is 109 cells, and the average number of degeneration ballooning in the various treatment groups ranged between 0 – 106 cells. While average number of degeneration ballooning in the negative control female group is 45 cells, and the average number of degeneration ballooning in the various treatment groups ranged between 0 – 160 cells.
Average number of kupfer cells in the negative control male group is 146 cells, and the average number of kupfer cells in the various treatment groups ranged between 102 – 510 cells. While average number of kupfer cells in the negative control female group is 192 cells, and the average number of kupfer cells in the various treatment groups ranged between 50 – 268 cells.
Average number of inflammation cells in the negative control male group is 41 cells, and the average number of inflammation cells in the various treatment groups ranged between 4-732 cells. While average number of inflammation cells in the negative control female group is 99 cells, and the average number of inflammation cells in the various treatment groups ranged between 7 – 185 cells.
Average number of necrosis cells in the negative control male group is 1 cells, and the average number of necrosis cells in the various treatment groups ranged between 0 – 12 cells. While average number of necrosis cells in the negative control female group is 3 cells, and the average number of necrosis cells in the various treatment groups ranged between 0 – 18 cells.
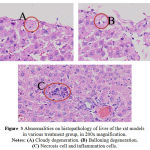 |
Figure 3: Abnormalities on histopathology of liver of the rat models in various treatment group, in 200x magnification. Notes: (A) Cloudy degeneration. (B) Balloning degeneration. (C) Necrosis cell and inflammation cells.
|
Discussion
Liver is the organ that is very important in maintaining body homeostasis, metabolism, biotransformation, synthesis, storage and immunology. Liver maintain body metabolism with very complex function and other essential process for life, such as energy storage, the production of proteins and bile, maintain cholesterol metabolism and toxic or drugs detoxification that enter the body (DEPKES RI, 2007). In the measurement liver function test, some enzymes that measured such as AST, ALT and ALP. Aspartic Acid aminotransferase (AST) and Alanine aminotransferase (ALT) is an enzyme that is contained in liver tissue, where if there is a damage in the liver AST and ALT levels will increase (Nyblom H et al, 2004).
Based on the data in this study suggest that the PSP treatment for 90 days (subchronic) in Rattus norvegicus Wistar strain rats, there is no effect on the function liver, this is shown from the result of measurement AST, ALT and ALP levels that show no significantly difference to the normal level.
AST levels in the blood is influenced by a liver, muscle, heart, brain and kidney condition, because AST contained in those organs. So if there is a damage or inflammation in liver and AST will go into blood circulation and cause the AST level in the blood increases. Based on research that has been done in Rattus norvegicus Wistar strain rats can be conclude that in both male and female treatment group, show that subchronic Ganoderma lucidum PSP treatment had not significantly effect in increasing AST levels in blood. This is appropiate with other study by Zhou X et al (2007) which mentioned that Ganoderma lucidum increase hepatoprotective to liver cause damage and can be used to treat the chronic hepatitis, and other study which mentioned that Polysaccharide peptide there was not any significant effect to liver (Dong Y et al, 1996). And there was not a significant effect on the two groups, male and female, this shows that Ganoderma lucidum PSP effects was not influenced by the hormones.
ALT levels in the bloods is influenced by liver condition, because liver contain the most amount of ALT, and small amount of ALT is contain in other organs, so ALT is more specific to teh liver. If there is a damage or inflammation in liver, ALT will go into blood circulation and cause the ALT levels in blood increases, where it is indicated if there is increasing level of ALT in blood we can assume that the liver was damaged. Based on research that has been done in Rattus norvegicus Wistar strain rats can be conclude that in both male and female treatment group, show that subchronic Ganoderma lucidum PSP treatment had not significantly effect in increasing AST levels in blood. This is appropiate with other study by Zhou X et al (2007) which mentioned that Ganoderma lucidum increase hepatoprotective to liver cause damage and can be used to treat the chronic hepatitis, and other study which mentioned that Polysaccharide peptide there was not any significant effect to liver (Dong Y et al, 1996). And there was not a significant effect on the two groups, male and female, this shows that Ganoderma lucidum PSP effects was not influenced by the hormones.
Alkaline phosphatase (ALP) is an enzyme that contained within the cell which is located on the bile from liver. The increase levels ALP happen if there are obstruction gall bladder disease, infiltrat on liver or intrahepatic cholestatis. ALP levels in the blood is influenced by liver dysfunction, duct bladder, or gallbladder. If there is a damage organs in it will cause increasing level of ALP in the blood, because the mucose cells on bile system from the liver is a source of ALP. Based on research that has been done in Rattus norvegicus Wistar strain rats can be conclude that in both male and female treatment group, show that subchronic Ganoderma lucidum PSP treatment had not significantly effect in increasing ALT levels in blood. This is appropiate with other study by Zhou X et al (2007) which mentioned that Ganoderma lucidum increase hepatoprotective to liver cause damage and can be used to treat the chronic hepatitis, and other study which mentioned that Polysaccharide peptide there was not any significant effect to liver (Dong Y et al, 1996). And there was not a significant effect on the two groups, male and female, this shows that Ganoderma lucidum PSP effects was not influenced by the hormones.
The results data of examination of liver function supported with the data obtained from the investigation histopathology organs liver, where a parameter that observed is the histopathology anatomy changes organs of Rattus norvegicus Wistar strain rats liver : cloudy degeneration, balloning degeneration, the number of cells kupfer per 10 spacious perspective on enlargement 40x, the number of inflammation cells per 10 spacious perspective on enlargement 40x, and necrosis cell tissue. From the observation result found that there has been abnormality in the liver tisuue such as cloudy degeneration and balloning degeneration that only happens in the peripheral area of liver tissue, and there are necrosis cells and the inflammation cells that only on small number of cells, while liver cells (hepatosit) that in a normal condition is still in a very much, and liver can still compensated that shown from the assessment liver function is still in the normal levels.
Conclussion
The effects of Ganoderma lucidum Polysaccharide peptide (PSP) treatment to liver can be proved by the result of AST, ALT, ALP levels and liver histopathology. The conclusion of this study is subchronic treatment of Ganoderma lucidum PSP from three dosages was not cause liver function changes or organ damages.
Acknowledgments
The authors would like to thanks to Ministry of Education and Culture, Indonesia. And to PT. Sahabat Lingkungan Hidup Mitra Lab Surabaya for their product that support our research.
References
- Cao QZ, et al. 2003. Antitumor and anti-angiogenic activity of Ganodermalucidum polysaccharides peptide.Acta Pharmacol Sin. 2004; 25(6):833-838
- Xu Z. et al. 2011. Ganodermalucidumpolysaccharides : immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39(1):15-27
- Silva D. 2003. Ganoderma lucidum (Reishi) in cancer treatment. Integr Cancer Ther.2003;2(4):358-64
- DEPKES RI. 2007. Pharmaceutical Care UntukPenyakitHati. Jakarta: DEPKES RI.
- Nyblom H, Berggren U, Balldin J, Olsson R (2004). High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol.39(4):336–339
- Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R (September 2006). The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int.26(7):840–845.
- Zhou X, Lin J, Yin Y, Zhao J, Sun X, Tang K. 2007. Ganodermataceae: natural products and their related pharmacological functions. Am J Chin Med. 2007; 35(4): 559-74
- Dong Y, Kwan CY, Chen ZN, Yang MM. 1996. Antitumor effects of a refind polysaccharide peptide fraction isolated from Coriolus versicolor: in vitro and in vivo studies. Res Commun Mol Pathol Pharmacol. 1996; 92(2): 140-8







