Padera Faryadyan1, Afra Khosravi2, Meysamkashiri3, Sarafaryadian4 and Naser Abasi5
1Psychosocial Injuries Research Centre, Ilam University of Medical Sciences, Ilam, Iran
2Department of Immunology , Faculty of Medicine, Ilam University of Medical Sciences,
3Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
4Psychosocial Injuries Research Centre, Ilam University of Medical Sciences, Ilam, Iran
5Department of Pharmacology, Faculty of Medicine Pease, Iran University of Medical Sciences, Tehran, Iran.
DOI : https://dx.doi.org/10.13005/bpj/467
Abstract
Background.Prenatal and postnatal stimulations have been revealed to have long-term effects on rat offspring various reproductive and behavioral reactions.The current study was designed to assay the impact of prenatal and postnatal exposure to different colors on variations of serum estrogen levels in female adult offspring. Four groups of pregnant female Wistar rats (seven rats in each) were enrolled in this study. The pregnant rats were placed in color chambers including green, blue, black and white (control). After delivery, female offspring’s were kept in color chambers until age 8-9 weeks, and then estrogen concentration in serum of female adult offspring’s were measured using the ELISA Kit. Our study result revealed that serum estrogen level increased in female adult offspringwho were prenatally and postnatally exposed to white and black colors compared to female adult offspring’s who were exposed to green and blue colors. Exposure to blue color significantly decreased serum estrogen level in female adult offspring. These findings demonstrated serious changes of estrogen in female offspring due to prenatal and postnatal exposure to different colors which can be translated as estrogen variables. We can document that prenatal and postnatal stimulation can lead to estrogen alterations in female offspring and such changes can be made by colors.
Keywords
Testosterone; Prenatal; Male offspring; Color; Rat; ElISA Kit
Download this article as:| Copy the following to cite this article: Faryadyan P, Khosravi A, Meysamkashiri, Sarafaryadian, Abasi N. Prenatal and Postnatal Exposure to White and Black Colors Increase Serum Estrogen Level in Female Rat Offspring. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Faryadyan P, Khosravi A, Meysamkashiri, Sarafaryadian, Abasi N. Prenatal and Postnatal Exposure to White and Black Colors Increase Serum Estrogen Level in Female Rat Offspring. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2889 |
Introduction
Estrogen is a steroid hormone whose synthesis takes place in the ovaries, adrenal cortex, and the placenta (Mesiano and RJaffe,1997; Villee and Tsai,1969; Siiteri and Serón-Ferré,1981). Estrogen adjusts embryogenesis, cell proliferation and differentiation, organogenesis, the timing of parturition, and fetal imprinting by carrying chemical messages from glands to cells within tissues or organs in the body (Jovana and Wendy, 2012). Steroid hormones become active as chemical mediators to evoke both slow genomic and rapid nogenomic responses and as a result modify essential cellular and physiological reactions. Steroid hormones’ action is vital for fetal growth and development (Mesiano and RJaffe,1997; Thompson and Yang, 2002). Among steroid hormones that secrete during pregnancy, estrogen is particularly important, because it is expressed at every stage of pregnancy and modifies many intrauterine processes (Blackburn, 2007; Ishimoto and Jaffe, 2011). A review of experimental studies on animals investigated the effects of social environment during pregnancy on the offspring’s’ behavior, brain, and neuroendocrine function and the results showed that stability of social environment during pregnancy in non-human mammals is vital for the offspring’s’ sexual and social behavior later in life as well as for reproductive activity, androgen and estrogen receptor distribution in particular parts of the brain and endocrine condition (Kaiser and Sachser, 2005). In addition, a study revealed that stress could change maternal care by modulatingthe neuroendocrine systems that adjust this behavior. “The effects of environmental adversity can be transmitted across generations through a nongenomic mechanism involving maternal care.” This study revealed enduring effect of gestational stress on both mother and maternal offspring in third mating (Champagne and Meaney, 2006). Exposure to stress during pregnancy provokes marked changes in the behavior of the affected offspring. A finding of a previous study suggests that exposure to stress during gestation significantly changed adult rats’ behavior (Koenig et al.,2005). Pregnant female rats and mice exposure to stress can greatly provoke alterations on the characteristics of female offspring’s, such as fertility and fecundity, age of vaginal opening and length of estrous cycles, and sex ratio of offspring’s (Herrenkohl, 1979; Herrenkohl and Politch, 1978; Politch and Herrenkohl, 1984. Kinsley and Svare, 1987), maternal behavior (Kinsley and Svare, 1987; Kinsley, 1988), sexual behavior (Allen and Haggett 1977), response to pain (Kinsley and Mann, 1988), brain function (Moyer et al., 1987), and pituitary function (Kinsley, 1987). A research assessing important effects of genetic and environmental factors on behavior indicated that there were no significant difference between such effects on males and females (Rhee et al., 2002). Some studies show that environmental factors can significantly determine and alter behavior (Stern, 2000). A study on rhesus monkeys revealed effectiveness of several social and environmental variables on circulating testosterone concentrations. Independent and interaction effects were displayed. The factors influencing concentrations of circulating testosteroneincluded successful and unsuccessful agonistic encounters, ontogenetic status, relative access to female’s circadian rhythms, season (seasonal rhesus typical mating) and alterations in social rank (Irwin et al., 1974). ). A study on the effects of prenatal social environment on brain, neuroendocrine function and offspring’s’ behavior has revealed that stability of the social environment during female gestation is vital for the offspring’s’ reproductive functioning, endocrine condition and androgen and estrogen receptor distribution in particular parts of the brain, and social and sexual behavior of offspring’s (Sylvia andNorbert, 2005). No studies have investigated the effects of prenatal exposure to different colors on changes of estrogen level in female offspring in human or animal models. In this study, we investigated the of effects prenatal and postnatal exposure to different colors as environmental factors on estrogen level changes of female rat adult offspring’s.
Animals
The experiment was conducted on four groups (seven animals each) of Wistar pregnant female rats weighed 200-250g. The rats were kept in a 25±2 °C temperature with a 12 hr light /dark cycle and fed with standard diet and tap drinking water. Rats were purchased from the animal house of Ilam University. All procedures were approved by the division of Animal house, as well as the ethical committee of Ilam University of Medal Sciences.
Procedure of prenatal exposure to different colors
Immediately after matching, four groups (seven animals each) of Wistar pregnant female rats were transferred to color chambers. The chambers were constructed of clear Plexiglas covered by different colors, including green, blue, black and white (control) colors (16 inches × 20 inches with 8-inch-high walls). For each chamber, the surrounding walls, floor and roofs were all painted in the same color as the chamber color according to the classification of working groups. After delivery, female offspring of all color groups8-9 weeks, when their serum samples were extracted.
Color wavelengths
The blue and green colors were analyzed with thin-layer chromatography (TLC) for quantitative wavelengths determinations. The wavelengths of blue and green colors were 450 and 490 nanometers, respectively as indicators of light color, and black color was indicator of dark color and the white color was control group.
Blood collection, hormone assay
The serum samples of all female offspring’s at age 8-9 weeks were collected for the measurement of estrogen according to the procedures previously described (Khosravi et al 2012, Hossenzadeh et al. 2012, Gholami parizad et al 2013, Direkvand-Moghadam et al 2012). Estrogen was assayed in each rat serum sample using the DRGinternational GMBH ELISA kit (Germany). The procedure of estrogen measurement is summarized below.
Assay estrogen
First, 25 μL of each standard, control and samples were added by new disposable tips into appropriate wells. Then 200 μL Enzyme conjugate was dispensed into each well and mixed for 10 seconds. Then it was incubated for 120 minutes at room temperature (without covering the plate). The next stage was briskly shake out the contents of the wells, following by rinsing the wells for 3 times with diluted Wash Solution (400 μL per well). Next stage was, striking the wells sharply on absorbent paper to remove residual droplets. Then 100 μL of Substrate Solution was added to each well and was incubated for 15 minutes at room temperature. In this stage the enzymatic reaction stop was provoked by adding 50 μL of stop solution to each well. The last stage was determined by absorbance (OD) of each well at 450±10 nm with a microtiter plate reader.
Statistical analysis
ANOVA was used to compare estrogen in each group of 7 rats. Normality of data in each group was checked using one sample kolmogroror- simirnov test. Dennett test was performed as a post hoc for multiple comparison analysis. P-values less than 0.05 were considered significant.
Result
Effect of prenatal exposure to different colors on changes of serum estrogen levels in female adult off spring’s
Figure 1 indicates the effect of prenatal exposure to different colors on changes of serum estrogen level in female adult offspring’s. ANOVA analysis indicated the differences between various groups (p < 0.05), and the following analysis with Dunnett- test (table 1) displayed that changes of serum estrogen level between female adult offspring’s who were pre and postnatally exposed to black and/or green color with adult male offspring’s who were exposed to white color were not statically significant. On the other hand, the results showed that the mean level of serum estrogen in female adult offspring’s who were pre and postnatal exposed to blue color with female adult offspring’s who were exposed to white color was statically higher (p < 0.05). The mean level of estrogen in female offspring’s exposed to black and/or green colors were significantly increased as compared with the group exposed to blue color. It can be seen that increasing amount of serum estrogen level was significantly high in female adult offspring who were pre and postnatal exposed to white color (Table1).
Table 1: The effect of Prenatal exposure to different colors on serum estrogen level changes in male adult off spring
| Dependent Variable | group | Mean | Std. Deviation | Std. Error | 95% Confidence
Upper Bound |
|
| white (control) | 161.96 | 8.983 | 3.395 | 170.26 | ||
| Serum estrogen | black | 150.57 | 12.791 | 4.834 | 162.40 | |
| blue | 134.91 | 9.377 | 3.544 | 143.59 | ||
| green | 149.06 | 10.454 | 3.951 | 158.73 | ||
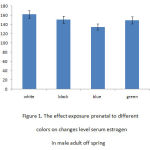 |
Figure 1: The effect exposure prenatal to differentcolors on changes level serum estrogen in male adult off spring |
Discussion
Our findings reveled significant increase in serum estrogen levels of female offspring’s who were exposed to white, black and green colors as compared to those who were pre and postnatally exposed to blue color. It can be seen the highest estrogen level was in white group while the lowest was in blue group. These results demonstrated that female offsprings’estrogen secretion was more sensitive to white and black colors as compared to blue color. Our findings showed that pre and postnatal exposure to colors could provoke significant changes in estrogen level of female offspring’s. They also indicated what colors could provoke more alterations in concentration of serum estrogen in female offspring’s.
Chronic exposure of teleost homologue to social stress induces chronic activation of the hypothalamic–pituitary–internal (HPI) axis, of the mammalian hypothalamic–pituitary–adrenal (HPA) axis (Winberg and Lepage, 1998; Øverli et al., 1999; Höglund et al., 2000). Alpha-melanocyte-stimulating hormone (α-MSH) and adrenocorticotrophic hormone (ACTH) are implicated in adjusting of internal cortisol release and inducing skin darkening (Balm et al., 1995; Fujii and Oshima, 1986).
The animal research showed magnify enhance in binding estrogen to protein content both hypothalamus and cortex in embryonic day 14 to postnatal day 15 in mouse , while estrogen receptor levels identifiable in embryonic day 13 which occur a surge in the perinatal period. This elevate possibly associated to the start of the phase of sexual differentiation known as ‘defeminization’ which appears to interfere estrogen receptors reaction to estradiol generated from testosterone and which provokes to be limited to the very early postnatal period((W.J. Friedman, McEwen, Toran-Allerand, & Gerlach,1983). The purpose of referring to above studies is to distinguish importance of time course in expression of estrogen and estrogen receptors during embryonic and postnatal period, which are important in investigating prenatal and postnatal exposure to environmental factors on changes of estrogen level.
To emphasize effect of environmental factors such as color lights, male and female meat chickens were exposed to blue, green, red, or white lights at 30 lx for 28 days and the effects on tissue growth and bird behavior was investigated. Birds were more active and aggressive in the red and white lights treatment, which resulted in weight loss after one week. Birds in all treatments indicated tendency for blue and green lights because they were calmer with blue and green light treatment (Prayitno, and Grimm, 1977). Color provokes an important cue for female attendance. Female rhesus monkeys have been demonstrated a preference for reddish-pink facial coloration of infant vervets compared to yellow or green. Female color preference was warmer colors than boys (i.e., pink and red) to cooler colors (i.e., blue and green) (Minamoto, 1985 cited in Iijima, Arisaka, Minamoto, & Arai, 2001). A preference for red or reddish pink has been proposed to stimulate female behaviors to infants which increase infant survival, such as contact (Higley, Hopkins, Hirsch, Marra, & Suomi, 1987).
Background matching is well known in fish, and a study manipulated body colors of a pair of fish and found that different environmental background colors of both fish who were exposed to white background was initially pale in coloration and they showed a high level of aggressive behavior. However, the frequency of aggressive interactions declined over time. In the pairs who were placed on a black background decreasing in aggressive behavior over time was less obvious than in pairs interacting on the white background (Abbott et al., 1985; O’Connor et al., 2003; Höglund et al., 2000).
Conclusion
Some studies show that fetuses exposed to environmental factors or pharmacological agents suffer from changes which suppress their normal gonadal function and result in immature sexual development and behavior in adulthood (Taché et al., 1980; Ward et al., 1994). According to our finding, pre and postnatal exposure to different color environments provokes different alterations between color groups. Mechanisms for induce of estrogen level changes due to different colors are complicated and unclear because unfortunately there has not been enough experimental studies on the effect of exposure to color during pregnancy on offspring’s’ different behaviors and development. So we can’t explain contributing mechanisms, and further investigation on this subject is necessary to discover secretes of color effects as enviromental factor during pregnancy on development or impairment of offspring’s’ behavior in animals and/or humans. We suggest further investigation of this subject on pregnant mother and their neonatal in human to identify interference pathways caused by color outcomes.
Reference
- Abbott, J. C., Dunbrack, R. L., & Orr, C. D. (1985). The interaction of size and experience in dominance relationships of juvenile steelhead trout (Salmo gairdneri). Behaviour,92, 241-253.
- Allen, T. O., & Haggett, B. N. (1977). Group housing of pregnant mice reduces copulatory receptivity of female progeny. Physiology & Behavior, 19(1), 61-68.
- Blackburn,s.t..(2007). “Reproductive and developmental processes,” in Maternal, Fetal and Neonatal Physiology, C. Jackson (Ed.), pp. 100–102, Philadelphia: Elsevier Health Sciences,
- Champagne, F. A., & Meaney, M. J. (2006). Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological psychiatry, 59(12), 1227-1235.
- Direkvand-Moghadam, A., Khosravi, A. The impact of a novel herbal Shirazi Thymus Vulgaris on primary dysmenorrhea in comparison to the classical chemical ibuprofen. (2012) Journal of Research in Medical Sciences, 17 (7), pp. 668-670. Cited 2 times.
- Friedman, W. J., McEwen, B. S., Toran-Allerand, C. D., & Gerlach, J. L. (1983). Perinatal development of hypothalamic and cortical estrogen receptors in mouse brain: methodological aspects. Developmental Brain Research, 11(1), 19-27.
- Gholami Parizad, E., Khosravi, A., Gholami Parizad, E., Sadeghifard, N., Ghafourian, S. Evaluation of chronic hepatitis B infection in patients with seronegative HbsAg (2012) Iranian Journal of Public Health, 41 (2), pp. 100-104. Cited 1 time. Herrenkohl, L. R. (1979). Prenatal stress reduces fertility and fecundity in female offspring. Science, 206(4422), 1097-1099.
- Herrenkohl, L. R., & Politch, J. A. (1978). Effects of prenatal stress on the estrous cycle of female offspring as adults. Experientia, 34(9), 1240-1241.
- Higley, J. D., Hopkins, W. D., Hirsch, R. M., Marra, L. M., & Suomi, S. J. (1987). Preferences of female rhesus monkeys (Macaca mulatta) for infantile coloration. Developmental psychobiology, 20(1), 7-18.
- Höglund, E., Balm, P. H. M., &Winberg, S. (2000). Skin darkening, a potential social signal in subordinate Arctic charr (Salvelinus alpinus): The regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J, Exp.,Biol. 203, 1711–1721.
- Iijima, M., Arisaka, O., Minamoto, F., &Arai, Y. (2001). Sex differences in children’s free drawings: a study ongirls with congenital adrenal hyperplasia. Hormones and Behavior, 40, 99–104.
- Irwin ,S., Bernstein, Robert, M., Rose, &Thomas, P., Gordon.(1974). Behavioral and environmental events influencing primate testosterone levels. Journal of Human Evolution,V 3( 6), Pages 517–525.
- Ishimoto, H., & Jaffe, R.B . (2011). Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocrine Reviews,32(3), 317–355.
- Khosravi, A., Ghafourian, S., Shamsi, M., Sadeghifard, N., Maleki, A., Babaahmadi, E. Cross-reaction between the crude hydatid cyst fluid antigens of human and animals origin in response to human IgG class and subclasses. (2012) Journal of Parasitology Research, 2012, art. no. 947948, . Cited 1 time.
- Khosravi, A., Hommel, M., Sayemiri, K. Age-dependent antibody response to Plasmodium falciparum merozoite surface protein 2 (MSP-2). (2011) Parasite Immunology, 33 (3), pp. 145-157. Cited 5 times.
- Koenig, J. I., Elmer, G. I., Shepard, P. D., Lee, P. R., Mayo, C., Joy, B., … & Brady, D. L. (2005). Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behavioural brain research, 156(2), 251-261.
- Jovana, Kaludjerovic.&Wendy, E. Ward. (2012). The Interplay between Estrogen and Fetal Adrenal Cortex. Journal of Nutrition and Metabolism, 2012 , pp,12, Review Article. Kinsley, C., & Svare, B.(1988). Prenatal stress alters maternal aggression in mice. Physiol Behav, 42:7-13.
- Kinsley, C., & Svare, B. (1987). Genotype modulates prenatal stress effects on aggression in male and female mice. Behavioral and neural biology, 47(2), 138-150.
- Kinsley, C.(1988). Bridges It Prenatal stress and maternal behavior in intact virgin rats: response latencies are decreased in males and increased in females. Horm Behav, 22:76-89.
- Kinsley, C., 7 Mann, P.(1988). Bridges It Prenatal stress alters morphine, and stress-induced analgesia in male and female rats. Pharm Biochem Behav. 30:123-128.
- Kinsley, C(1987). Bridges It Prenatal stress reduces estradiol-induced prolactin release in male and female rats. Physiol Behav. 40:647-653.
- Kaiser, S., & Sachser, N. (2005). The effects of prenatal social stress on behaviour: mechanisms and function. Neuroscience & Biobehavioral Reviews,29(2), 283-294.
- Mesiano,s., & Jaffe,R.B.(1997). Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews,18( 3), 378–403.
- Minamoto, F. (1985). Male–female differences in pictures. Tokyo: Shoseki. Moyer, L., Herrenkohl, L., & Jacobowitz, D.(1987). Stress during pregnancy: effect on catecholamines in discrete brain regions of oıprmg as adults. Brain Res,144, 173- 178.
- O’Connor, T.G., Heron, J., Golding, J., & Glover, V .(2003). Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry, 44, 1025–1036.
- Politch, J., Herrenkohl, L.(1984). Effects of prenatal stress on reproduction in male and female mice. Physiol Behav, 32,95-99.
- Rhee, Soo Hyun., &Waldman, Irwin, D .(2002).Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies, Psychological Bulletin, Vol 128(3), 490-529
- Siiteri,P.K., & Serón-Ferré,M.(1981). “Some new thoughts on the fetoplacental unit and parturition in primates.in Fetal Endocrinology. M. J. Novy and J. A. Resko, Eds., pp. 1–34, Academic Press, New York, NY, USA.
- Stern, P. C. (2000). New environmental theories: toward a coherent theory of environmentally significant behavior. Journal of social issues, 56(3), 407-424.
- Tache, Y., Ducharme, J.R., Charpenet, G., Haour, F., Saez, J.,& Collu, R.(1980). Effect of chronic intermittent immobilization stress on hypophyso-gonadal function of rat. Records Endocr. Copenh. 93,168-174.
- Thompson,A. V. K. M. Han, &Yang,K.(2002). “Spatial and temporal patterns of expression of 11β-hydroxysteroid dehydrogenase types 1 and 2 messenger RNA and glucocorticoid receptor protein in the murine placenta and uterus during late pregnancy. Biology of Reproduction, 67( 6), 1708–1718.
- Villee,A.,& Tsai,S.C.(1969). The de novo synthesis of steroids by the placenta,” in The Foetal-Placental Unit, A. Pecile and C. Finzi, Eds., pp. 110–119, Excerpta Medica Foundation, Amsterdam, The Netherlands.
- Ward, I.L., Ward, O.B., Winn, R.J.,& Bielawski, D.(1994). Male and female sexual behavior potential of male rats prenatally exposed to the influence of alcohol, stress or both factors. Behav Neurosci,108,1188-1195.
- W.J. Friedman, B.S. McEwen, C.D. Toran-Allerand1.,&J.L. Gerlach.(1983) Perinatal development of hypothalamic and cortical estrogen receptors in mouse brain: Methodological aspectsDevelopmental Brain Research, 11(1), 19–27








