Manuscript accepted on :12 Sep 19
Published online on: 30-09-2019
Plagiarism Check: Yes
Reviewed by: Frank Ngonda
Second Review by: Dini Damayanti
Final Approval by: Dr. Maria Anastasiadou
Shreeraksha Upadhyaya1, Shivaprakash Gangachannaiah1* and Pallavi Lakshmi Chandrashekar2
and Pallavi Lakshmi Chandrashekar2
1Department of Pharmacology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India-576104.
2Department of Physiology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India-576104.
Corresponding Author E-mail: shiva.g@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/1771
Abstract
Alzheimer's disease is a common neurological disorder affecting a significant proportion of the elderly population.There are only a few drugs which can be used safely to treat it. We sought to investigate for the first time Piper betel leaf, a postmeal mouth freshener for its potential use in Alzheimer's disease. Five groups of male Wistar rats with six rats in each group aged 10-12 weeks were used. The control group received the distilled water, aluminium chloride (AlCl3) group was treated with AlCl3, the standard group was treated with rivastigmine with AlCl3, and the two test groups received piper betel leaf extract (PBE) at doses of 400mg/kg and 500mg/kg body weight with AlCl3. AlCl3 was administered orally for 42 days in all the treated groups. Memory and learning were evaluated by Morris water maze test and Passive avoidance test.The results of the Morris water maze test showed reduced mean escape latency period in all the groups on trial day three (P≤0.05) and on trial day four (P ≤0.01) compared to AlCl3 group. The retention of spatial memory by probe trial showed that PBE and rivastigmine treated group spent more time in the target (platform) quadrant when compared to AlCl3 group (P≤0.01). The passive avoidance test showed a significant increase in step through latency in standard and test groups compared to the AlCl3 treated group. The weight of the rats treated with AlCl3 and PBE was reduced at the end of the treatment period while increased in standard and control group. The study shows the beneficial effects of Piper betel leaves in Alzheimer’s disease by significantly improving the learning and memory functions in rats.
Keywords
Alzheimer’s Disease; Learning; Memory; Piper Betel Leaves
Download this article as:| Copy the following to cite this article: Upadhyaya S, Gangachannaiah S, Chandrashekar P. L. Effect of Piper betel leaf extract on learning and memory in Aluminium chloride induced Alzheimer’s disease in Wistar rats. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Upadhyaya S, Gangachannaiah S, Chandrashekar P. L. Effect of Piper betel leaf extract on learning and memory in Aluminium chloride induced Alzheimer’s disease in Wistar rats. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28689 |
Introduction
Alzheimer’s disease (AD) is the common cause of dementia, manifested as impaired memory and disturbances in cognitive functions. Its pathology is characterized by the accumulation of A-beta peptide, which is formed by the action of enzyme secretases on amyloid precursor protein (APP).1 According to Alzheimer’s Association Report 2017, an estimated 5.5 million Americans have AD and is projected to grow to 13.8 million in 2050.2 In India, 3.7 million people aged more than 60 years were estimated to be suffering from dementia and is expected to double by 2030.3
Treatment of AD includes six FDA approved drugs – tacrine, rivastigmine, galantamine, donepezil, memantine and memantine with donepezil combination. These drugs being mainly cholinergic drugs were found to have modest beneficial effects on cognition and memory, do not reverse the memory loss, have a brief duration of action, and are associated with adverse effects due to activation of the peripheral cholinergic system. Memantine has potential to cause neurotoxic effects if not given for long periods when combined with anticholinesterase drugs.4 Moreover, in 2005, NHS expressed skepticism on the ability of the AD drugs to meaningfully improve quality of life or delay hospitalizations.5
Betel leaves were used as a postmeal mouth freshener and also popular for its medicinal uses in India, Srilanka, Malaysia, Thailand, Taiwan and other southern asian countries.6 Betel leaves contain many neuroprotective and cognition-enhancing chemical components like hydroxychavicol, caryophyllene, quercetin.7 In-vitro studies have proved betel leaves ability to inhibit a beta-secretase enzyme which catalyzes the production of senile plaque in AD.8 Hydroxychavicol (HC) at graded doses administered orally for 21 days in rats improved the cognition and reduced pro-inflammatory cytokines. It attenuated the lipid peroxidation and activities of both β- and γ-secretase enzymes.9 Beta-caryophyllene was shown to reduce oxidative stress and demyelination process in multiple sclerosis in a murine model. Its cannabinoid (CB2) receptor agonism was shown to improve cognition and to reduce neuroinflammation.10 However, to date, none of the studies to the best of our knowledge has tested the role of betel leaves extract in the AD. Hence, we sought to evaluate its role in the animal model of the AD.
Materials and Methods
Preparation of aqueous betel leaf extract
The leaves were obtained from the local market in Udupi. The leaf was authenticated by Dr M. S. Joishni Kumble, Department of Botany, Canara College, Mangaluru, Karnataka, India. A voucher specimen (CC/Cert./2017-2018) has been deposited in the herbarium of the Canara College, Mangaluru University, Karnataka, India under the license No.CC-Jan/2018/01. The 100g of Piper betel leaves was grounded with 150ml of distilled water in a grinder and kept at 4ºC for 24 hours. The extract is filtered under vacuum and used for this study.
Dosage: Earlier studies have shown that LD₅₀ value 3g/kg of betel leaf extract to be non- toxic to rats.11, 12 Two different oral doses of aqueous leaf extract (400mg/kg and 500mg/kg) of piper betel were used in the present study.
Drugs and Chemicals: Aluminium chloride was purchased from Merck Life Sciences Private Limited (Vikhroli East, Mumbai, India) and rivastigmine was procured from Novartis Pharmaceuticals Ltd (Worli, Mumbai, India).
Animals: Male Wistar rats weighing 200–225 g, aged 10-12 weeks, procured from institutional Central Animal House and maintained at 12/12 h light/dark cycle, 25±2 ºC temperature and 60 % humidity. Standard pelletized feed and water were provided ad libitum. The experiment was conducted after getting approval by the Institutional Animal Ethics Committee (IAEC/KMC/125/2016).
Experimental Design
The following five groups of male Wistar rats with six rats in each group were used:
Control: Rats were administered with distilled water by the oral route.
AlCl3: Rats were treated with AlCl3 (100 mg/kg body weight orally) for 42 days.
Standard: Rats were treated with AlCl3 as group II for 42 days and Rivastigmine(5 mg/kg)
(one hour before AlCl3) from day 21 to 42 days by oral gavage.
PBE 400mg: Rats were administered AlCl3 like group II for 42 days and PBE (400mg/kg)
(one hour before AlCl3) from day 21 to 42 days by oral gavage.
PBE 500mg: Rats were administered AlCl3 like group II for 42 days and PBE (500mg/kg)
(one hour before AlCl3) from day 21 to 42 days by oral gavage.
At the end of the experiment, animals were tested for memory by Passive avoidance test and Water Maze Test.
Passive avoidance Test 13
This is a widely used method for memory testing. The apparatus consists of two compartments (26×28×16cm each), with one illuminated and the other dark chamber, separated by an automatically operated guillotine door. The dark compartment is provided with an electrifiable grid, which is connected to a constant current stimulator. The test was conducted
for 2 consecutive days at the same time each day. On the first day (acquisition trial) each rat was placed in the illuminated compartment facing away from the dark compartment and allowed to explore freely. The rats were allowed to enter the dark compartment. Once the rat enters completely into the dark compartment, the door is closed immediately and given an electric shock (50 Hz, 1.5 mA, and 1 s duration) at 5-second intervals. After the acquisition trial, twenty-four hours later, the same experiment was repeated for retention trial but without administering an electric shock. When the rats stayed in the light compartment, it was considered positive memory retention and impaired if it enters the dark compartment. The maximum cut-off latency time was set at 300 sec.
Morris Water Maze
Spatial learning and memory abilities were assessed according to Vorhees and Williams, with minor modification.14 Rats were tested in a Morris water maze (diameter of 150cm and depth of 29cm, 57 cm high, 23°C±2°C).The escape platform is made invisible by adding milk powder to the pool. Throughout the experiment, the platform was concealed just below the water surface (2 cm) in a fixed place in one of the four divided quadrants of the pool. During the training period, rats have to find the concealed platform using distal cues. One day before the test, the rats were provided free swim in the pool without a platform for 60seconds to enable each rat to become accustomed to the environment. On days one- to- four, rats were trained for 16 trials (four trials a day) to find and escape onto the immersed platform. The latency time to escape to the hidden platform in the pool was noted during each trial. Every trail began by releasing the animal from different positions into the maze, facing towards the wall of the pool to search for the platform to a maximum cutoff 60 seconds. Within the given time if the rat did not escape to the platform, it was guided to the platform and allowed to remain there for 15s. The time to reach the platform was recorded using a video camera. The probe trial was done on day 5, 24hr following the training trails on day four. In the probe trial, the platform was removed from the pool and each rat was allowed to swim for 60 seconds. The time spent in the target quadrant within that 60seconds of probe test time was recorded. This estimates the retention of spatial memory.
Statistical Analysis: Statistical analysis was performed by one-way analysis of variance followed by Tukey test and student paired t-test wherever applicable using Statistical Package for the Social Science software package version 20.0 (IBM Corp: Armonk, NY). Results were expressed as a mean ± Standard error means for each group. Probability values (P) ≤0.05 was considered statistically significant.
Results
Table 1: Mean body weight in different groups.
| Body Weight | Control | AlCl3 | Standard | PBE400mg | PBE500mg |
| Day1 | 143±14 | 168±5 | 164±8 | 170±7 | 165±8 |
| Day42 | 175±19 | 163±4 | 177±8 | 160±3 | 159±6 |
| P | 0.007 | 0.011 | 0.002 | 0.03 | 0.01 |
Values are expressed as mean±Standard error of mean.P≤0.05 is considered significant. PBE: Piper betel leaf extract; AlCl3: Aluminium chloride
Body weight changes in all the groups were shown in Table 1. The body weight was found to increase in control and standard group after the treatment period. The AlCl3 group and PBE groups exhibited a decrease in body weight.
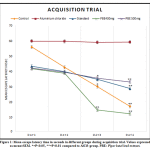 |
Figure 1: Mean escape latency time in seconds in different groups during the acquisition trial. |
The mean escape latency period was reduced in all the groups on trial day 3 (P≤0.05) and day 4 (P ≤0.01) compared to AlCl3 group. The AlCl3 group exhibited an increase in escape latency on all four days to find the platform. There was a downward trend in time latency with all the groups as days advances, except for AlCl3 treated group (Figure 1).
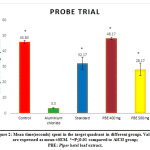 |
Figure 2: Mean time(seconds) spent in the target quadrant in different groups. |
The probe trial demonstrated that the time spent in the platform (target) quadrant was lesser with Aluminium chloride group compared to all the other groups. This indicates lesser retention of spatial memory on exposure to aluminium. PBE co-treatment significantly attenuated (P≤0.01) the impairment of memory caused by AlCl3 (Figure 2).
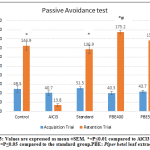 |
Figure 3: Effect of PBE on step-through type passive avoidance test. |
Learning and memory was assessed by performing passive avoidance test. In the acquisition trial, there was no significant difference in step-through latency time among experimental groups (Figure 3). However, during retention trial, the latency was dramatically decreased in the AlCl3 rats, and AlCl3-reduced step-through latency was effectively restored by PBE administration at 400mg/kg and 500mg/kg (Figure. 3).There was a significant increase in step-through latency in PBE 400 group compared to the standard group.
Discussion
The current study was undertaken to evaluate the effects of piper betel leaves in AD. Two doses of betel leaf extract were selected based on previous studies.11,12 The results showed, lose of weight in aluminium (Al) and PBE treated groups and weight gain in rivastigmine and control groups (Table 1). Al administration is known to reduce the desire for food intake in rats.15 Similar to our study Abdul Gani et al. showed, in rats administered with PBE at 500mg/kg leads to a reduction in food intake in high fat fed rats.16 A randomized, double-blind controlled trial was reported to cause weight loss with PBE by rising serum levels of adiponectin and decrease in ghrelin peptide.17 Studies have shown relationship between weight loss and improvement in memory.18 Similarly in our study there was loss of weight in PBE groups. This to an extent might have contributed to its learning and memory enhancing activity. However, further studies are required to find its exact mechanism responsible for improvement in learning and memory in AD.
Learning and memory impairment are common manifestations with the AD. There is still no effective treatment to reverse and restore the dysfunctional nervous system in the AD. A naturally grown and consumed agent, which can restore the neuronal dysfunction with minimal side-effects and cost will be valuable. Aluminium Chloride salts when administered to rats, causes neurotoxicity by multifaceted action. It accumulates in the hippocampus(site of learning and memory) by entering the brain via transferrin receptors.19 It affects learning and memory by increasing AChE activity, increased accumulation of beta-amyloid and reducing the antioxidant activity.20,21 In the present study, AlCl3 treated group has impaired memory in both water maze test and in passive avoidance test compared to other groups indicating impaired learning and memory (Figure 1, 2 and 3).
PBE group showed significant improvement in learning by exhibiting decreased escape latency time as days advances during the acquisition trial. (Figure 1)The group also showed greater memory retention by spending more time in platform quadrant in probe trial and increased step-through latency in passive avoidance test compared to AlCl3 treated group.(Figure 2 and 3) PBE contains many neuroprotective phytoconstituents. Dalai et al., reported by an in-vitro study that hydroxychavicol and chlorogenic acid from extracts of betel leaves inhibited both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes significantly.22 Both these enzymes decrease the acetylcholine (ACh) levels in AD causing cholinergic dysfunction. Pandey et al. demonstrated that chronic oral treatment of hydroxychavicol (HC) extracted from Piper betle in rats at graded doses reduced the concentration of both enzymes β- and γ-secretases.9 The Aβ region of the APP is cleaved by these proteases, which are responsible for the accumulation of Aβ in the brain.23 Allylopyrocatechol, a phytoconstituent of Piper betle, was demonstrated by De et al., to induce antioxidant enzymes catalase, superoxide dismutase and enhance reduced glutathione (GSH) levels by activation of the Nrf2 pathway.24 These multiple mechanisms may be responsible for the protective effects of PBE against Al-induced AD. Future studies should explore on underlying molecular mechanisms involved in the beneficial effects of PBE in the AD. However, this is the first study to establish successfully the useful effects of Piper betel leaves in rats induced with Alzheimer’s disease.
Conclusion
The study proves the learning and memory enhancing activity of Piper betel leaves in an animal model of Alzheimer’s disease. It can be useful in the human population and can be a potential area for future research.
Conflict of Interest: None declared.
Funding Source: None declared
Reference
- Cam JA, Bu G. Modulation of Beta-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol Neurodegener. 2006;1:8.
- Association A. 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement [Internet]. Elsevier Inc.; 2017;13:325–73.
- Summary E. Shaji KS, Jotheeswaran AT, Girish N, Srikala Bharath, Amit Dias, Meera Pattabiraman, Mathew Varghese, eds. Alzheimer’s and Related Disorders Society of India. The Dementia India Report: prevalence, impact, costs and services for dementia. New Delhi, India. Dementia. 2010;1–38.
- Wollen KA. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern Med Rev. 2010;15:223–44.
- Casey DA, Antimisiaris D, Brien JO. Drugs for Alzheimer ’ s Disease : Are They Effective ? Pharm Ther. 2010;35:208–11.
- Das S, Parida R, Sandeep IS, Nayak S, Mohanty S. Asian Paci fi c Journal of Tropical Medicine prospects. Asian Pac J Trop Med. 2016;9:938–46.
- Dwivedi V, Tripathi S. Review study on potential activity of Piper betle. J Pharmacogn Phytochem JPP. 2014;3:93–8.
- Matsumura S, Murata K, Yoshioka Y, Matsuda H. Search for beta-Secretase Inhibitors from Natural Spices. Nat Prod Commun. 2016;11:507–10.
- Pandey A, Bani S. Hydroxychavicol inhibits immune responses to mitigate cognitive dysfunction in rats. J Neuroimmunol. 2010;226:48–58.
- Cheng Y, Dong Z, Liu S. Beta-caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 mice through CB2 receptor activation and the PPAR?? pathway. Pharmacology. 2014;94:1–12.
- S. Milton Prabu, M. Muthumani, K. Shagirtha. Protective effect of Piper betle leaf extract against cadmium-induced oxidative stress and hepatic dysfunction in rats. Saudi Journal of Biological Sciences. 2012; 19:229–239.
- L.S.R. Arambewela a, L.D.A.M. Arawwawala a, W.D. Ratnasooriya. Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. Journal of Ethnopharmacology 102 (2005) 239–245.
- King RA, Glasser RL. Duration of electroconvulsive shock-induced retrograde amnesia in rats. Physiol Behav. 1970;5:335–9.
- Vorhees C V, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58.
- Abdel-Aal RA, Assi AAA, Kostandy BB. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur J Pharmacol. 2011;659:169–76.
- Abdul Ghani ZDF, Husin JM, Rashid AHA, Shaari K, Chik Z. Biochemical studies of Piper betle L leaf extract on obese treated animal using 1H-NMR-based metabolomic approach of blood serum samples. J Ethnopharmacol.2016;194:690–7.
- Sengupta K, Mishra AT, Rao MK, Sarma KV, Krishnaraju A V., Trimurtulu G. Efficacy of a herbal formulation LI10903F containing Dolichos biflorus and Piper betle extracts on weight management. Lipids Health Dis. Lipids in Health and Disease; 2012;11:176.
- Boraxbekk CJ1, Stomby A, Ryberg M, Lindahl B, Larsson C, Nyberg L, Olsson T. Diet-Induced Weight Loss Alters Functional Brain Responses during an Episodic Memory Task.Obes Facts 2015;8:261–272
- Roskams AJ, Connor JR. Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci U S A. 1990;87:9024–7.
- Lin W-T, Chen R-C, Lu W-W, Liu S-H, Yang F-Y. Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’s disease rat model. Sci Rep. 2015;5:9671.
- Vasudevaraju BP, Govindaraju M, Palanisamy AP, Sambamurti K, Rao KSJ. Molecular toxicity of aluminium in relation to neurodegeneration. Indian J Med Res. 2008;128:545–56.
- Dalai MK, Bhadra S, Bandyopadhyay A, Mukherjee PK. Evaluation of anti-cholinesterase activity of the standardized extract of Piper betel L. leaf. Orient Pharm Exp Med. 2014;14:31–5.
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509.
- De S, Manna A, Kundu S, De Sarkar S, Chatterjee U, Sen T, et al. Allylpyrocatechol Attenuates Collagen-Induced Arthritis via Attenuation of Oxidative Stress Secondary to Modulation of the MAPK, JAK/STAT, and Nrf2/HO-1 Pathways. J Pharmacol Exp Ther. 2017;360:249–59.







