Gehan A. Hegazy1, 2, Olfat Shaker3, Safaa Sayed4, Amr Abd Elzaher5, Khaled Fathy5, Iman Wahby6,7, Ayman Elsamanoudy1,8 and Hesham N. Mustafa9
1Clinical Biochemistry Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
2Medical Biochemistry Department, National Research Centre, Cairo, Egypt.
3Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Cairo University, Egypt.
4Rheumatology and Rehabilitation Department, Cairo University, Cairo, Egypt.
5Internal Medicine Department, Ain Shams University, Cairo, Egypt.
6Family and Community Medicine Department, Rabigh, King Abdul Aziz University, Saudi Arabia.
7Community and Occupational Health Department, Al Azhar University, Faculty of Medicine, Egypt.
8Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
9Anatomy Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
Corresponding Author E-mail: hesham977@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1750
Abstract
Systemic Lupus Erythematosus (SLE) and systemic sclerosis (SSc) are systemic inflammatory autoimmune disorders characterized by a large spectrum of clinical and laboratory features. The aim of the present study was to investigate the possible use of serum level of soluble intercellular adhesion molecule-1(sICAM-1) and soluble interleukin-2 receptor (sIL-2Ra) as biomarkers for monitoring of SLE and SSc disease activity. Moreover, it aimed to compare the specificity and sensitivity as well as cut-off value of both biomarkers in a sample of Egyptian patients. 50 SLE patients, 30 SSc patients and 60 age and sex matched healthy controls were enrolled in our study. sICAM-1and sIL-2Ra were measured in serum samples obtained from all participants. In addition to Erythosedimentation rate (ESR), complete blood count (CBC), Antineuclearantibodies (ANA) estimation, disease activity of both diseases were also assessed. sICAM-1and sIL-2Ra levels were higher in SLE and SSc patients versus control. Both parameters are correlated with each other as well as the activity parameters. A cut-off levels of 455.59 (ng/ml) &2525935 (pg/ml) in both SLE & SSs respectively was observed with the highest specificity and sensitivity. It could be concluded that sICAM-1 and sIL-2Ra are noninvasive biomarkers for SLE and SSc that could play a pathophysiologic role in development and progression of both diseases. Moreover, sICAM-1 and sIL-2Ra are correlated with the disease activity at cut-off values of 455.59 (ng/ml) & 2525935(pg/ml) respectively.
Keywords
Soluble Intracellular Adhesion Molecule-1; Soluble Interleukin 2 Receptor; Systemic Lupus Erythematosus; Systemic Sclerosis
Download this article as:| Copy the following to cite this article: Hegazy G. A, Shaker O, Sayed S, Elzaher A. A, Fathy K, Wahby I, Elsamanoudy A, Mustafa H. N.Biomarkers of Systemic Lupus Erythematosus and Systemic Sclerosis diseases activity in a sample of Egyptian patients :Soluble Intercellular Adhesion Molecule-1 and Soluble Interleukin-2 Receptor, Case Control Study.Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Hegazy G. A, Shaker O, Sayed S, Elzaher A. A, Fathy K, Wahby I, Elsamanoudy A, Mustafa H. N.Biomarkers of Systemic Lupus Erythematosus and Systemic Sclerosis diseases activity in a sample of Egyptian patients :Soluble Intercellular Adhesion Molecule-1 and Soluble Interleukin-2 Receptor, Case Control Study.Biomed Pharmacol J 2019;12(3).Available from: http://biomedpharmajournal.org/?p=28423 |
Introduction
Connective tissue diseases (CTDs) are systemic inflammatory autoimmune disorders characterized by a large spectrum of clinical and laboratory features. CTDs spectrum includes Systemic Lupus Erythromatosus (SLE) and Systemic Sclerosis (SSc) 1. SLE is a chronic, multi-organ, relapsing autoimmune disease mainly affecting young adults. It is commoner among female, with the female/male ratio being approximately 9/12.
SSc is an uncommon chronic systemic connective tissue autoimmune disease characterized by microvascular abnormalities and pathological thickening and tethering of the skin and involvement of internal organs (gastrointestinal tract, heart, lungs, and kidneys). SSc seems to result from a multifactorial process (alterations of the immune system, genetic and environmental factors) but its pathogenesis remains unclear 3.
Autoantibodies/auto reactive T cells can attack any organ of the body, resulting in a wide array of signs and symptoms. Soluble molecules have been detected in a broad range of surface proteins, which include T cell antigens such as (CD8), adhesion molecules such as intercellular adhesion molecule-1 (sICAM-1) and cytokine receptors such as the alpha chain of the high affinity interleukin-2 receptor(sIL-2R). Soluble receptors are suggested to compete with the corresponding cellular receptors for ligands and thus inhibiting ligands action 4.
Therefore, it was suggested that receptor shedding may be useful non-invasive markers for CTDs activity, especially in both SLE and SSc patients and also correlated with disease activity 5. IL-2 deficiency affects multiple regulatory pathways in the host and in the case of SLE, this contributes to the multifaceted dysregulation of the immune response. The interleukin-2 receptor a (IL-2Ra) chain is a component of high-affinity IL-2 receptors and thus is a key regulator of lymphocyte proliferation 5.
In SSc, it was found that the severity of the disease is correlated with sIL-2Rand those with early onset had the highest sICAM-1 levels 6. Although the role and functions of soluble ICAM-1 and IL-2Ra have not yet been completely elucidated, the evidence suggests its implication in disease progression, or at least its elevated levels may inform the clinician about pathological processes associated with SLE and SSc.
Therefore, the aim of the present study was to investigate the possible use of serum level of sICAM-1 and sIL-2Ra as biomarkers for monitoring of SLE and SSc disease activity. Moreover, it aimed to compare the specificity and sensitivity as well as cut-off value of both biomarkers in a sample of Egyptian patients.
Subjects and Methods
Sampling
Sample was calculated by EPI program with confidence 95%, power 85%, OR=5 and it was 77 participants in each group. So, cases were elevated to 80 (30 SSc and 50 SLE), and due to deficiency in the controls which were matched to the cases, and another who refused the sharing in the study, the controls were decreased to 60 participants.
The present case control study was performed on 140 subjects’ enrolled equal from Rheumatology outpatient clinics and internal medicine departments of Cairo and Ain Shams University Hospitals during period from May 2016 to August 2018 according to the principles of the Helsinki Declaration. A Local ethics committee of the faculty of Medicine of Cairo and Ain Shams Universities approved the study.
An informed consent was obtained from all subjects participating in this study, after explaining its nature. The subjects were classified into 3 groups. Group I consist of 50 patients with SLE fulfilling the ‘American College of Rheumatology (ACR)’ criteria for diagnosis of SLE 7. Group II consists of 30 patients with SSc fulfilling the ACR criteria for diagnosis of SSc 8. Group III included age and sex matched 60 apparently healthy volunteers and served as control group.
All patients were not under immunosuppressive therapy at the time of enrolment. After taking their consent, all participants were subjected to full history taking, general, and local and skin examination.
Biochemical assessment
Ten milliliters venous blood samples were obtained from all participants via venipuncture. Five milliliters of them were placed into plain tubes and allowed to clot for 15 minutes. Sera were prepared by centrifugation at 1,500 g for 10 min, aliquoted and stored at – 80 ºC for the following investigation; CRP levels were measured by the turbidimetric method using a photometer (Biosystems S.A., Barcelona, Spain), and a level of <6 mg/L was accepted as normal and creatinine were measured by the ordinary commercial available kits.
Antinuclear antibody (ANA) was measured by indirect immune-fluorescence supplied by Immco Diagnostics (USA).Three milliliters were collected in K2 EDTA tubes for complete blood count (Coulter STKS hematology flow cytometer, Block Scientific, Inc., Bohemia, New York, USA).The last two milliliters were collected in Sodium citrate tubes for measurement erythrocyte sedimentation rate (ESR). ESR was measured by using the Westergren method and expressed in mm/h 9.
Moreover, all subjects were asked to collect 24 hours urine samples for quantitative measurement of 24 hours urinary proteins and complete urine analysis.
Quantikine Human sICAM-1and sIL-2Ra (Cat.Number DY720-05; DR2A00 respectively-USA R&D Systems, Inc. 614 McKinley Place NE, Minneapolis, MN 55413) immunoassay kits were a solid phase ELISA designed to measure serum sICAM-1 and sIL-2Ra respectively. Results obtained using natural human sICAM-1and sIL-2Ra showed linear curves that were parallel to the standard curves obtained using the Quantikine kit standards.
Statistical analysis
Statistical Science for Social Package (SPSS version 20, SPSS Inc., Chicago, IL, USA) was used for data analysis. Data were expressed as mean ± SD for quantitative data, and frequency with its percentage with graphs of qualitative data. One-way ANOVA test was used for comparison parametric parameters and Chi square test for non-parametric parameters between different groups.
The Pearson’s and Spearman correlation coefficient tests were used to evaluate associations between measured parameters and non-parametric parameters. The area under the receiver operating characteristic curve (AUC-ROC) analysis was performed to determine sensitivity and specificity of both sICAM-1and sIL-2Ra as biomarker indicator test to SLE and SSc diseases activity. For all tests, P <0.05 was considered significant.
Results
All participants in this study were 140. The majority (75.0%) of them were female, while the males represent only 25%. Also, 50 (35.71%) from them had Systemic lupus erythromatosis, 30 (21.43%) had Systemic Sclerosis and; 60 (42.86%) were the controls ( table 1).
Table 1: Demographic Characteristics and Disease Durations of all Studied Groups.
|
Studied Sample
Demographic Characteristics: |
Systemic lupus erythromatosis (SLE) | Systemic Sclerosis
(SSc) |
Controls | Significant Tests
P-value |
|||
| No. | % | No. | % | No. | % | ||
| Number | 50 | 35.71% | 30 | 21.43% | 60 | 42.86% | |
| Sex:
· Female · Male |
42 8
|
84.0% 16.0% |
23 7 |
76.7% 23.3% |
40 20
|
66.7% 33.3% |
0.11
|
| Age (years): | 44.68± 10.47 | 47.31 ± 12.04 | 41.58 ± 11.89 | 0.07 | |||
| Disease duration (years): | 7.97±3.44 | 5.93±2.55 | – | 0.00* | |||
Data are expressed as mean ± standard deviation or number (%) as appropriate. P: significance versus control; *P: significance versus SSc used One-way ANOVA test (Post Hoc test).
The demographic characteristics and disease duration of all participants are presented in table (1). Females were more than males in SLE (84.0% versus 16.0%), in SSc (76.7% versus 23.3%) and in controls (66.7% versus 33.3%) with statistically insignificant difference between groups (P=0.11). In addition, there were also statistically insignificant differences between the studied groups in relation to age (P =0.07) but the duration of disease was significantly longer in SLE than SSC (P =0.00).
Table (2) illustrated the clinical and some laboratory characteristics in different studied groups. The arthritis, kidney diseases and heart diseases were more common in SLE than SSc patients were (72%, 36% and 16% versus 53%, 20% and 13%, respectively, P>0.05). Antinuclear antibodies had a high percentage among SLE compared to SSc patients (92% versus 73%, P <0.05). On the opposite side, lung diseases occur more in SSc patients compared to SLE (50% facing to 6%, respectively, P<0.05). On the other hand, neurologic diseases and proteinuria occur only among the SLE patients (8% and 54% respectively, P<0.05) while, myositis occurs only among SSc patients (13.3%, P<0.05). Meanwhile, there was no statistically significant between the studied groups in relation to present of serosities or measurement of C-reactive protein (P>0.05).In table (3), ESR was significantly higher in SLE and SSc versus control (76.88±45.40 and 65.73±72.61 versus 10.80±3.56, P<0.000). Also, serum creatinine was significant higher in SLE than SSc and control (2.09±0.44 versus 0.85±0.24 and 0.76±0.19, P <0.0001). While, Leucocytic Count showed insignificant difference in SLE, SSc and control groups (6.30 ± 1.40, 6.55±1.77; and 6.23±1.83,respectively. P =0.696).
Table 2: Clinical and Some Laboratory Characteristics in Different Studied Groups
| Studied Sample
Clinical Characteristics: |
Systemic lupus erythromatosis
(SLE) (n=50) |
Systemic Sclerosis
(SSc) (n=30) |
Significant Tests Ӽ2-test |
||
| No. | % | No. | % | ||
| Systemic involvement
Arthritis |
36 |
72% |
16 |
53.3% |
0.09 |
| Serositis (pleurisy only) | 4 | 8% | 6 | 20% | 0.11 |
| Kidney | 18 | 36% | 6 | 20% | 0.13 |
| Heart | 8 | 16% | 4 | 13% | 0.75 |
| Lung | 3 | 6% | 15 | 50% | 0.00* |
| Neurological | 4 | 8% | 0 | 0% | 0.00* |
| Myositis | 0 | 0% | 4 | 13.3% | 0.00* |
| Proteinuria positive | 27 | 54% | 0 | 0% | 0.00* |
| Antinuclear antibodies +ve | 46 | 92% | 22 | 73.3% | 0.02* |
| C-reactive protein +ve | 19 | 38% | 11 | 36.7% | 0.91 |
Data are expressed as number (%) as appropriate. P: significance versus control; *P: significance versus SSc used One-way ANOVA test (Post Hoc test).
Table 3: Some Laboratory Investigations in Different Studied Groups.
| Studied Sample
Laboratory Characteristics |
Systemic lupus erythromatosis
(SLE) (n=50) |
Systemic Sclerosis
(SSc) (n=30) |
Controls
(n=60) |
Significant Tests
P-value |
| mean ± SD | mean ± SD | mean ± SD | ||
| Erythrocytic Sedimentation rate (mm/hour) | 76.88±45.40 | 65.73±72.61 | 10.80±3.56 | 0.00 |
| Leucocytic Count (x 103/mm3) | 6.30 ± 1.40 | 6.55±1.77 | 6.23±1.83 | 0.69 |
| Serum Creatinine (mg/dl) | 2.09±0.44 | 0.85±0.24 | 0.76±0.19 | 0.00* |
Data are expressed as mean ± standard deviation (mean ± SD). P: significance versus control; *P: significance versus SSc used One-way ANOVA test (Post Hoc test).
Comparison of serum levels of sICAM-1 and sIL-2R in different studied groups is presented in table (4). sICAM-1 level was higher in SLE and SSc patients versus control (498.80±205.90 and 437.62±172.52 versus 158.49±43.31, P <0.000). The same result was recorded in sIL-2R that was higher in SLE and SSc patients versus control (3.002.76 ± 749.12 and 2.167.24±509.43 versus 848.23±68.78, P <0.000) and these differences were statistically significant between SLE versus SSc (P<0.000).
Table 4: Comparison of Serum Levels of Soluble Intracellular Adhesion Molecules-1 (sICAM-1) and Soluble Interleukin 2 Receptor (sIL-2R) in Different Studied Groups.
| Studied Sample
Variables |
Systemic lupus erythromatosis
(SLE) (n=50) |
Systemic Sclerosis
(SSc) (n=30) |
Controls
(n=60) |
Significance Tests
P-value |
| mean ± SD | mean ± SD | mean ± SD | ||
| sICAM-1 (ng/ml) | 498.80±205.90 | 437.62±172.52 | 158.49±43.31 | 0.000 |
| sIL-2R (pg/ml) | 3.002.76 ± 749.12 | 2.167.24±509.43 | 848.23±68.78 | 0.000* |
Data are expressed as mean ± standard deviation (mean ± SD). P: significance versus control; *P: significance versus SSC used One-Way ANOVA test (Post Hoc test).
Correlations of sICAM-1 and IL-2Ra with the measured parameters in SLE are illustrated in table (5). sICAM-1 shows strong, moderate or even week correlation the other main biochemical parameters (IL-2Ra) [positive correlation]. Moreover, sICAM-1 shows a correlation with cutaneous limitation, subcutaneous activity, photosensitivity, oral ulcer, discoid rash [positive correlation], molar rash and proteinuria [negative correlation]. While, it shows no correlation with the other measured parameters.
Table 5: Correlations between sICAM-1/ sIL-2R and Measured Parameters in Systemic Lupus Erythromatosis Patients.
| Parameter
|
sICAM-1 | sIL-2R | |||
| r | P-value | r | P-value | ||
| 1. Sex | 0.049 | 0.515 | 0.139 | 0.334 | |
| 2. Age | 0.098 | 0.497 | 0.214 | 0.136 | |
| 3. Duration | -0.049 | 0.733 | 0.174 | 0.226 | |
| 4. Arthritis | 0.284 | 0.046 | -0.030 | 0.837 | |
| 5. Serositis (pleurisy only) | -0.016 | 0.912 | 0.161 | 0.912 | |
| 6. Kidney | 0.076 | 0.600 | -0.264 | 0.064 | |
| 7. Heart | 0.126 | 0.382 | 0.106 | 0.464 | |
| 8. Lung | 0.228 | 0.111 | -0.019 | 0.897 | |
| 9. Neurological | 0.057 | 0.696 | -0.157 | 0.277 | |
| 10. Cutaneous limitation | 0.248 | 0.083 | 0.150 | 0.298 | |
| 11. Subcutaneous activity | 0.341 | 0.015 | 0.341 | 0.515 | |
| 12. Photosensitivity | 0.151 | 0.296 | 0.316 | 0.025 | |
| 13. Malar rash | -0.244 | 0.088 | -0.078 | 0.588 | |
| 14. Oral ulcers | 0.141 | 0.328 | -0.021 | 0.885 | |
| 15. Discoid rash | 0.190 | 0.185 | -0.120 | 0.407 | |
| 16. ESR | 0.199 | 0.166 | -0.203 | 0.157 | |
| 17. C Reactive protein | -0.079 | 0.588 | 0.383 | 0.037 | |
| 18. Leucocytic count | 0.187 | 0.194 | -0.115 | 0.428 | |
| 19. Serum creatinine | -0.095 | 0.511 | 0.492* | 0.000 | |
| 20. Antinuclear antibody | 0.174 | 0.226 | 0.004 | 0.977 | |
| 21. Proteinuria | -0.104 | 0.472 | -0.277 | 0.052 | |
| 22. sIL-2R | 0.132 | 0.360 | 1 | 1 | |
The correlation between parametric parameters was made using Person correlations.
The correlation of sICAM-1 and IL-2Ra with the measured parameters in SSc is presented in table (6). sICAM-1 and IL-2Ra are strongly correlated with each other and with Raynaud s phenomenon, gastrointestinal affection, myositis and skin scars [positive correlation. While, they are negatively correlated with Antinuclear antibodies as well as each other. No correlation is detected with the other measured parameters.
Table 6: Correlations between sICAM-1/ sIL-2R and measured parameters in Systemic Sclerosis patients.
| Parameter
|
sICAM-1 | sIL-2R | |||
| r | P-value | r | P-value | ||
| 1. Sex | 0.363 | 0.045 | -0.136 | 0.474 | |
| 2. Age | 0.045 | 0.812 | -0.153 | 0.418 | |
| 3. Duration | 0.191 | 0.31 | -0.305 | 0.101 | |
| 4. Raynaud s phenomenon | 0.019 | 0.922 | 0.204 | 0.279 | |
| 5. Gastrointestinal | 0.067 | 0.723 | -.0103 | 0.590 | |
| 6. Arthritis | -0.659* | 0.000 | 0.338 | 0.068 | |
| 7. Kidney | 0.021 | 0.912 | 0.021 | 0.912 | |
| 8. Heart | 0.019 | 0.920 | -0.067 | 0.726 | |
| 9. Lung | -0.070 | 0.714 | -0.344 | 0.062 | |
| 10. Myositis | 0.285 | 0.127 | -0.280 | 0.134 | |
| 11. Skin score | 0.162 | 0.39 | 0.379 | 0.392 | |
| 12. ESR | 0.088 | 0.642 | -0.303 | 0.104 | |
| 13. C Reactive protein | -0.060 | 0.755 | 0.383 | 0.037 | |
| 14. Leucocytic count | 0.092 | 0.627 | -0.112 | 0.555 | |
| 15. Serum creatinine | -0.003 | 0.986 | -.0158 | 0.404 | |
| 16. Antinuclear antibody | -0.409* | 0.025 | 0.451* | 0.012 | |
| 17. sIL-2R | 0.511* | 0.00 | 1 | 1 | |
The correlation between parametric parameters was made using Person correlations.
Regrading ROC analysis, cut off levels of both sICAM-1 and IL2Ra are 455.59 (ng/ml) &2525935(pg/ml) in both SLE & SSs respectively. with the highest sensitivity and specificity.The cut-off levels as well as area under curve for both are presented in graphs (1& 2).
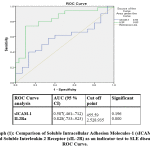 |
Graph 1: Comparison of Soluble Intracellular Adhesion Molecules-1 (sICAM-1) and Soluble Interleukin 2 Receptor (sIL-2R) as an indicator test to SLE disease, ROC Curve. |
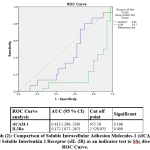 |
Graph 2: Comparison of Soluble Intracellular Adhesion Molecules-1 (sICAM-1) and Soluble Interleukin 2 Receptor (sIL-2R) as an indicator test to SSc disease, ROC Curve. |
Discussion
In the current study, there were significant increase level of sICAM- in SLE patients was higher than that in healthy control as reported by Egerer et al. 10; Sabry et al. 11;Kluz et al. 12. In addition, the mean level of sICAM-1 in SSc was higher compared to healthy controls. A finding previously reported by Hasegawa et al. 13.
Circulating sICAM-1 has been considered as the result of proteolytic cleavage of cell-bound ICAM-1 close to the cell membrane. ICAM-1 cleavage is regulated by tumor necrosis factor-a-converting enzyme and multiple kinases, including mitogen-activated protein kinase, S locus receptor kinase, and phosphoinositide 3-kinase pathways 13.
SLE pathophysiologic events are mediated by formation of immune complexes and complement cascade activation with progressive tissue destruction and major organ damage complication like lupus nephritis and cardiovascular morbidity. On basis of evidences , inflammation plays a major role in SLE induced vascular dysfunction and consequently associated morbidity and mortality 11.
Cytokines are known to be produced by inflammatory cells. Endothelial cell adhesion molecules could play a pathophysiologic role in the initiation and progression of autoimmune diseases as well as atherosclerosis. ICAM-1, VCAM-1, and E-selectin are the major adhesion molecules that are mostly induced by the increased proinflammatory cytokines. They are detected by tissue immunohistochemistry in atherosclerotic lesions in animal as well as human studies reported by Sabry et al. 11.
Inflammation, endothelial dysfunction, and atherosclerosis are interlinked pathological events. All share a common pathogenesis process. Elevated serum sICAM-1 is an evident biomarker and considered as indirect indicator for SLE induced tissue damage 14.They reported sICAM-1 tissue up-regulation. Recently in 2017, Gensous et al. 15 concluded that s ICAM-1 is an evident biomarker for both SLE pathogenesis and disease progression.
At the level of genetic study, ICAM polymorphisms have been studied and linked to their serum levels as well association with SLE risk and disease activity. rs3093030 and G in rs5498 alleles of sICAM-1 gene were found to be linked to SLE susceptibility. The genotype–phenotype relationships are evident and could explain the raised plasma levels of soluble ICAM-1 in patients with SLE and its underlying etiological role 16.
Previous studies described sICAM-1 as a potential biomarkers.sICAM-1 may provide information about the endothelial cells integrity and dysfunction in SSc 17 but it lacks specificity to SSc 18 and it is contradictory up to date 19.
There are some reports demonstrating the critical roles of these biomarkers in SSc patients or animal model of SSc 20. sICAM-1 is functionally active and retains the ability to inhibit leukocyte endothelial cell interaction. On the other hand, sICAM-1 has also been reported to promote angiogenesis and induce the production of TNF-α, IFN-γ, IL-6, and macrophage inflammatory protein-2. Thus, sICAM-1 may also have proinflammatory potential 21. In 2010 Yoshizaki et al. 22 suggested that L-selection and ICAM-1 regulate Th2 and Th17 cell accumulation into the skin and lung, leading to the development of fibrosis, and ICAM-1 deficiency inhibited the development of dermal sclerosis and pulmonary fibrosis with decreased inflammatory cell infiltration in the bleomycin-induced SSc model 23, 22.
In accordance with the results of the present study, IL-2R was also seen on lymphocytes (CD30) of SLE and SSc diseases 24. Their expression indicates a regulatory role for these molecules in SLE and SSc. Shedding of soluble IL-2R molecules into the circulation is the result of proteolytic cleavage of the membrane bound molecules. Other previous studies have reported that IL-2R levels were higher in patients with SLE than that in controls 25, 26.Serum sIL-2R is a reliable marker of disease activity in patients with SLE and could be used as an indicator of early renal involvement with the possibility of using it for follow-up 27. Swadzba et al. 28 reported that increased level of sIL-2R is connected with definite SLE where inflammatory processes prevail 28.
Klonowska-Szymczyk et al. 29 revealed higher concentrations of sIL-2R in the cell culture in the active phase of SLE in their study. They explained this by that cells from patients with active SLE have marked response to stimulation by TLR3 and TLR9 ligands. The higher response and increased sIL-2R may be related to greater cell reactivity and involvement of the receptors of lymphocyte activation. They also, confirmed presence of significant correlation sIL-2R concentration and the activity of lupus nephritis 29. Moreover, Dejica 26 reported that sIL- 2R concentration (marker of lymphocyte activation) positively correlates with SLE induced renal inflammation.
At the level of genetic study, Carr et al. 30 proved association of IL2RA locus with SLE .They explain this by that soluble IL-2RA concentrations correlate with rs11594656 genotype in SLE 30, 31.
Regarding the positive correlation that is detected between sICAM and sIL-2Ra in both SLE and SSc as autoimmune diseases, This correlation was previously reported in multiple sclerosis as another example of autoimmune diseases by Witkowska et al.32. The significant correlation of sICAM-1 and most of SLE disease activity that are observed in the current study confirm a nearly similar results of Sari et al., 33. They reported a significant positive correlation between sICAM-1 serum levels and SLE disease activity index (SLEDAI) score in SLE patients in their study. They also concluded that sICAM-1 serum level measurement and follow up may be considered as an important serologic marker of disease activity for assessment a patient with SLE.
Many previous studies demonstrated a positive correlation of sIL-2R with SLE disease activity as it flares with active course of the disease and decreases with therapy and clinical improvement. These studies are mentioned in a review written by Illei et al.34. So, sIL-2R levels paralleled lupus disease activity as observed in our study. Findings that confirm our observation related to the correlation of both parameters with systemic sclerosis disease activity were found as Mittag et al. 35 regarding sICAM-1 and Witkowska et al 32 regarding sIl-2Ra.
To the best of our knowlage, it is the first study that investigate the cut off value with the highest specificity and sensitivity of both sICAM-1 and sIL-2Ra that can be used as abiomarker for mentoring the disease activity in both SLE and SSc in Egypt.The levels of 455.59 (ng/ml) &2525935(pg/ml) for sICAM-1 and IL-2Ra respectively could be considered as remarkerable value of both diseases which need further multicentric investigation over larger samples to confirm the results of the current study. From the results of the current study, it could be concluded that sICAM-1 and sIL-2Ra are noninvasive biomarkers for SLE and SSc that could play a pathophysiologic role in development and progression of both diseases. Moreover, sICAM-1 and sIL-2Ra are correlated with the disease activity at cut-off values of 455.59 (ng/ml) &2525935(pg/ml) respectively.
Limitation of the current study could that the patients were recruited from the inpatient wards and outpatient clinics of Cairo and Ain-Shams Universities Hospitals. That is considered unicenter study. Moreover, the small number of selected patients that are enrolled in our study especially SSc patients.
In conclusion, the authors recommended that further multicentric studies should be done on a large number of SLE and SSc patients to evaluate and confirm our hypothesis in the role of ICAM-1 and sIL2-Ra as markers in Systemic Lupus Erythematosus and Systemic Sclerosis. Then clinician may ask for both ICAM-1 and Soluble Interleukin-2 receptor levels to monitor the disease activity and the response of their patients to the treatment.
Acknowledgments
Nothing
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding source
Nothing
References
- Spagnolo P, Cordier JF, Cottin V. Connective tissue diseases, multimorbidity and the ageing lung. Eur Respir J 47 1535-1558. (2016)
- Housey M, et al. Incidence and prevalence of systemic lupus erythematosus among Arab and Chaldean Americans in southeastern Michigan: the Michigan Lupus Epidemiology and Surveillance Program. American journal of public health 105 e74-79. (2015)
- El Basel M, Khalil N. Disease characteristics of systemic sclerosis among Egyptian patients. Kasr Al Ainy Medical Journal 21 41. (2015)
- Falcini F. Vascular and connective tissue diseases in the paediatric world. Lupus 13 77-84. (2004)
- Italiani P, et al. IL-1 family cytokines and soluble receptors in systemic lupus erythematosus. Arthritis Res Ther 20 27. (2018)
- Vettori S, et al. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 39 485-489. (2010)
- Tedeschi SK, et al. Developing and Refining New Candidate Criteria for Systemic Lupus Erythematosus Classification: An International Collaboration. Arthritis Care Res (Hoboken) 70 571-581. (2018)
- van den Hoogen F, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis and rheumatism 65 2737-2747. (2013)
- Bedell SE, Bush BT. Erythrocyte sedimentation rate. From folklore to facts. The American journal of medicine 78 1001-1009. (1985)
- Egerer K, et al. Increased serum soluble CD14, ICAM-1 and E-selectin correlate with disease activity and prognosis in systemic lupus erythematosus. Lupus 9 614-621. (2000)
- Sabry A, et al. Intercellular adhesion molecules in systemic lupus erythematosus patients with lupus nephritis. Clin Rheumatol 26 1819-1823. (2007)
- Kluz J, Kopec W, Jakobsche-Policht U, Adamiec R. Circulating endothelial cells, endothelial apoptosis and soluble markers of endothelial dysfunction in patients with systemic lupus erythematosus-related vasculitis. Int Angiol 28 192-201. (2009)
- Hasegawa M, et al. Serum adhesion molecule levels as prognostic markers in patients with early systemic sclerosis: a multicentre, prospective, observational study. PloS one 9 e88150. (2014)
- Zaccagni H, Fried J, Cornell J, Padilla P, Brey RL. Soluble adhesion molecule levels, neuropsychiatric lupus and lupus-related damage. Front Biosci 9 1654-1659. (2004)
- Gensous N, et al. Predictive biological markers of systemic lupus erythematosus flares: a systematic literature review. Arthritis Res Ther 19 238. (2017)
- Kim K, et al. Variation in the ICAM1-ICAM4-ICAM5 locus is associated with systemic lupus erythematosus susceptibility in multiple ancestries. Ann Rheum Dis 71 1809-1814. (2012)
- Castro SV, Jimenez SA. Biomarkers in systemic sclerosis. Biomark Med 4 133-147. (2010)
- Hummers LK. Microvascular damage in systemic sclerosis: detection and monitoring with biomarkers. Curr Rheumatol Rep 8 131-137. (2006)
- Oleszowsky M, Seidel MF. Serum Soluble Vascular Cell Adhesion Molecule-1 Overexpression Is a Disease Marker in Patients with First-Time Diagnosed Antinuclear Antibodies: A Prospective, Observational Pilot Study. BioMed research international 2018 8286067. (2018)
- Hasegawa M, Takehara K. Potential immunologic targets for treating fibrosis in systemic sclerosis: a review focused on leukocytes and cytokines. Seminars in arthritis and rheumatism; 2012: Elsevier; 2012. p. 281-296.
- Shaker O, et al. Role of ICAM-1 and E-selectin gene polymorphisms in pathogenesis of PAOD in Egyptian patients. Vasc Health Risk Manag 6 9-15. (2010)
- Yoshizaki A, et al. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. Journal of immunology 185 2502-2515. (2010)
- Rehberger P, Beckheinrich-Mrowka P, Haustein UF, Sticherling M. Prostacyclin analogue iloprost influences endothelial cell-associated soluble adhesion molecules and growth factors in patients with systemic sclerosis: a time course study of serum concentrations. Acta Derm Venereol 89 245-249. (2009)
- Rocha-Parise M, et al. Lymphocyte activation in silica-exposed workers. Int J Hyg Environ Health 217 586-591. (2014)
- Davas E, et al. Serum IL-6, TNFα, p55 srTNFα, p75 srTNFα, srIL-2α Levels and Disease Acitivity in Systemic Lupus Erythematosus. Clinical rheumatology 18 17-22. (1999)
- Dejica D. Serum soluble IL-2 receptor as a marker of lymphocyte activation in some autoimmune diseases. Effect of immunosuppressive therapy. Roumanian archives of microbiology and immunology 60 183-201. (2001)
- El-Shafey EM, El-Nagar GF, El-Bendary AS, Sabry AA, Selim AG. Serum soluble interleukin-2 receptor alpha in systemic lupus erythematosus. Iran J Kidney Dis 2 80-85. (2008)
- Swadzba J, Iwaniec T, Musial J. Increased level of tumor necrosis factor-alpha in patients with antiphospholipid syndrome: marker not only of inflammation but also of the prothrombotic state. Rheumatol Int 31 307-313. (2011)
- Klonowska-Szymczyk A, et al. The impact of agonists and antagonists of TLR3 and TLR9 on concentrations of IL-6, IL10 and sIL-2R in culture supernatants of peripheral blood mononuclear cells derived from patients with systemic lupus erythematosus. Postepy Hig Med Dosw (Online) 71 867-875. (2017)
- Carr EJ, et al. Contrasting genetic association of IL2RA with SLE and ANCA-associated vasculitis. BMC Med Genet 10 22. (2009)
- Makino T, Jinnin M. Genetic and epigenetic abnormalities in systemic sclerosis. J Dermatol 43 10-18. (2016)
- Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm 2005 121-130. (2005)
- Sari RA, et al. Correlation of serum levels of soluble intercellular adhesion molecule-1 with disease activity in systemic lupus erythematosus. Rheumatol Int 21 149-152. (2002)
- Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis and rheumatism 50 2048-2065. (2004)
- Mittag M, Beckheinrich P, Haustein UF. Systemic sclerosis-related Raynaud’s phenomenon: effects of iloprost infusion therapy on serum cyokine, growth factor and soluble adhesion molecule levels. Acta Derm Venereol 81 294-297. (2001)








