Mansour Haddad
Department of Clinical Sciences, Faculty of Pharmacy, Philadelphia University, Jordan. P.O box 3341, Irbid, Jordan P.C 211-10.
DOI : https://dx.doi.org/10.13005/bpj/445
Abstract
Urinary tract infection (UTI) is one of the most common infections in both outpatients and hospital patients. In the majority of the cases, antimicrobial treatment is usually started empirically before the laboratory results of the urine culture are available. Thus, antimicrobial resistance may increase in uropathogens due to frequent and improper use of antimicrobial agents. This study was conducted to identify the urinary pathogens of UTI, to determine the antimicrobial resistance pattern in UTI and to assess the choices available for empirical antimicrobial therapy in patients with UTI over a three-year period. This study was conducted at King Abdulla University Hospital, Irbid in the north of Jordan from 2003 to 2005. A retrospective analysis of data taken from all midstream urine samples (8,800 patients suspected of UTI) was analyzed and an antimicrobial susceptibility test for commonly usable antimicrobial agents was performed for the isolates using the standard disc diffusion method. Data was analyzed using SPSS software window version 12. The most frequent causative organisms isolated were Escherichia coli (48.2%), Klebsiella pneumoniae (14.5%), Pseudomonas aeruginosa (9.7%), Enterococcus faecalis (5.8%), and Proteus mirabilis (3.2%). Nitrofurantoin was found to be the most effective antimicrobial agent against E. coli, Klebsiella pneumonia and Enterococcus faecalis isolates. Gentamycin and ciproflaxcin were found to be the most effective antimicrobial agents against Pseudomonas aeruginosa. The progressive increase in antimicrobial resistance among patients with UTI is of great concern. Empirical antimicrobial selection should be based on knowledge of the local prevalence of bacterial organisms and antimicrobial sensitivities, because antimicrobial resistance patterns may vary in different regions. The findings of this study indicate that E. coli is the predominant pathogen of UTI. Moreover, nitrofurantoin should be considered for empirical therapy of lower tract UTI.
Keywords
Antimicrobial agents; Urinary tract infection; Susceptibility; Uropathogens; Escherichia coli
Download this article as:| Copy the following to cite this article: Haddad M. Antimicrobial Resistance of Uropathogens and Rationale for Empirical Therapy in Jordan. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Haddad M. Antimicrobial Resistance of Uropathogens and Rationale for Empirical Therapy in Jordan. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2818 |
Introduction
Antimicrobial agents have played an indispensable role in reducing the incidence of morbidity and mortality associated with infectious diseases in humans. However, the selective pressure on using antimicrobial agents was the main driving force behind the emergence and spread of antimicrobial resistance traits among bacterial pathogens (Farrell et al., 2003; Huang et al., 2014; Shaifali et al., 2012; Wang et al., 2014; Zhanel et al., 2006).
Urinary tract infection (UTI) is one of the most common infectious diseases in community practice diagnosed in patients with high financial cost (Haller et al., 2004; Kurutepe et al., 2005). Early diagnosis and prompt antimicrobial treatment are required to decrease renal scarring and prevent progressive kidney damage (Filiatrault et al., 2012). Indeed, antimicrobial therapy in patients with suspected UTI is usually started empirically before urine culture results are available. Unfortunately, antimicrobial resistance has become an increasingly pressing problem in many countries (Kiffer et al., 2011; Sire et al., 2007).
The antimicrobial susceptibility of urine pathogens, changing over the years, is influenced by such factors as the changing patient population, and the extensive use and misuse of antimicrobial agents, which contribute to alterations in the microbial profile of urine isolates (Karaca et al., 2005). Therefore, the overuse of antimicrobial agents may increase the risks of antimicrobial resistant pathogens, side effects and the cost of medical care (Usluer et al., 2005). Indeed, antimicrobial resistance is a growing problem that gives rise to major concerns (Wise et al., 1998). Moreover, there are large geographical variations in the patterns of bacterial resistance properties depending on local practices of antimicrobial prescriptions. Thus, different centres consider the results of a urine culture previously obtained from the laboratory of microbiology in determining empirical antimicrobial treatment for UTI.
Reporting the antimicrobial susceptibility testing of the urinary tract is usually achieved within 48 hours following the taking of a sample and, therefore, the treatment decision in the majority of UTIs is often empirical. The selection of antimicrobial agents is influenced by available data reflecting antibiotic resistance (Kurutepe et al., 2005). Since the initiation of antimicrobial therapy in UTI is empirical, knowledge of the antimicrobial resistance patterns of common uropathogens is essential to provide clinically appropriate and cost effective therapy and achieve both a favourable clinical outcome and a reduction in antimicrobial resistance (Kurutepe et al., 2005; Usluer et al., 2005). As accurate information about prescribing patterns in hospitals is valuable in improving the quality of antimicrobial prescriptions (Usluer et al., 2005), there will be a great need for antimicrobial resistance surveillance at the local, national and international levels (Kurutepe et al., 2005).
For a better understanding of the emergence of resistance since the advent of the era of antimicrobial agents, leading to the baseline data in the first years of testing at King Abdulla University Hospital (KAUH), UTI collections were assayed from human sources obtained during 2003–2005 for antimicrobial agent susceptibility. This information will give a broader picture of the development of resistance and lay the foundation for understanding the genetic mechanisms of resistance development.
The aim of this study was to determine the antimicrobial susceptibility patterns for most common strains of bacterial urine isolates taken from patients at KAUH in Irbid/the northern part of Jordan, from 2003 to 2005, and to evaluate the results. Therefore, the optimal empirical antimicrobial therapy for such patients could be determined in our region.
Study Design and Methods
Study design
A retrospective analysis was performed on all bacterial urine samples isolated from inpatients and outpatients sent to the bacteriology laboratory at KAUH for culture and sensitivity testing over three years, 2003, 2004 and 2005. Cultures with Candida growth were excluded from this analysis.
Susceptibility testing
The sensitivity of bacterial urine isolates to commonly used antimicrobial agents from different groups was determined using the standard disc diffusion method. The antibiotics used were gentamycin, nitrofurantoin, cefuroxime, ampicillin, trimethoprim-sulfamethoxazole, ceftriaxone, ciprofloxacin, tetracycline, amoxicillin-clavulanic acid, imipenem, cefazolin and chloramphenicol.
Statistical analysis
All data were analysed with SPSS for Windows, version 12.0, identified by percentage.
Results
A summary of the different urine bacterial microorganisms isolated from patients at KAUH in Irbid/the northern part of Jordan during the study period is shown in Table 1.
As expected, in 2004 E. coli (48.2%) was the leading uropathogen followed by Klebsiella pneumonia (14.5%), Pseudomonas aeruginosa (9.7%), Enterococcus faecalis (5.8%) and Proteus mirabilis (3.2%). Other bacteria were responsible in total for only 18.6% of positive urine cultures. For the other years (2003 and 2005), almost the same spectrum was found; E. coli (43.5 % and 43.9%, respectively) was the leading uropathogen followed by Klebsiella pneumoniae (18.5% and 13.7%, respectively), Pseudomonas aeruginosa (11.3% and 9.2%, respectively), then followed here by Proteus mirabilis (5.1% and 5.3%, respectively) and Enterococcus faecalis (4.5% and 3.4%, respectively). Other bacteria were responsible in total for only 17.1% and 24.4% of positive urine cultures respectively (Table 1).
Table 1: Prevalence (number and percentage) of bacterial urinary tract infections from patients at King Abdullah University Hospital in Irbid/the northern part of Jordan 2003-2005.
| Isolates
|
2003 | 2004 | 2005 | |||
| Percent | Frequency | Percent | Frequency | Percent | Frequency | |
| Escherichia coli | 43.5 | 594 | 48.2 | 43.9 | 1530 | |
| Klebsiella pneumoniae | 18.4 | 252 | 14.5 | 572 | 13.7 | 476 |
| Pseudomonas aeruginosa | 11.3 | 154 | 9.7 | 385 | 9.2 | 321 |
| Proteus mirabilis | 5.1 | 70 | 3.2 | 126 | 5.3 | 186 |
| Enterococcus faecalis | 4.5 | 62 | 5.8 | 230 | 3.4 | 120 |
| Others | 17. 1 | 234 | 18.6 | 734 | 24.4 | 851 |
| Total | 100 | 1366 | 100 | 3950 | 100 | 3484 |
Antimicrobial resistance profiles of bacterial urinary isolates, at KAUH in Irbid/the northern part of Jordan from 2003 to 2005, were summarized in Table 2. Antimicrobial resistance patterns from different groups for E. coli isolates, from patients at KAUH in Irbid/the northern part of Jordan from 2003 to 2005, were also presented in Figure 1.
Generally, the most effective antibiotic for the E. coli isolates observed was nitrofurantoin followed by amoxicillin/clavulanate and cephalosporins. Regarding E. coli, trimethoprim/sulfamethoxazole susceptibility (yearly range, 60 to 61%), nitrofurantoin susceptibility (yearly range, 6 to 9%) and gentamicin (yearly range, 35 to 41%) were generally unchanged over the 3 years studied. Tetracycline was the only agent studied that demonstrated a consistent stepwise increase in resistance from 2003 (57%) to 2005 (65%). Resistance rates to ampicillin (yearly range, 75 to 91%), ciprofloxacin (yearly range, 50 to 65%), amoxicllin/calvulinic acid (yearly range, 21 to 45%), and chloramphenicol (yearly range, 24 to 67%) varied over this 3-year period.
Although almost 91% of the E. coli isolates in 2003 were resistant to ampicillin, only 7% in 2004 were resistant to nitrofurantoin. From 2003 to 2005, more than 50%, 60% and 57% of the E. coli isolates were resistant to ciprofloxacin, trimethoprim/sulfamethoxazole and tetracycline, respectively. Also, a wide range of resistance patterns to cephalosporins (Cefazolin, Ceftriaxone, Cefuroxime) was observed.
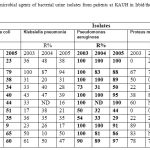 |
Table 2: Percentage of resistance to the antimicrobial agents of bacterial urine isolates from patients at KAUH in Irbid/the northern part of Jordan, from 2003 to 2005 (ND=Not Determined)(R=resistance).
|
 |
Figure 1: Antimicrobial resistance patterns in E.coli isolates from patients at KAUH in Irbid/the northern part of Jordan from 2003 to 2005. |
A wide range of antimicrobial resistance patterns was observed for Proteus mirabilis and Klebsiella pneumoniae. From 2003 to 2005, more than 64% and 47% of these bacterial urinary isolates were resistant to ampicillin and trimethoprim/sulfamethoxazole, respectively. Relatively, lower resistance of ciprofloxacin, nitrofurantoin and cefazolin was observed for Klebsiella pneumoniae while lower resistance of ciprofloxacin, ceftriaxone, cefuroxime and amoxicillin-clavulanic acid was observed for Proteus mirabilis.
As shown in Table 2, almost all antimicrobial agents were highly resistant to Pseudomonas aeruginosa, and the relatively susceptible antimicrobial agents were ciprofloxacin, ceftriaxone, gentamycin for these bacteria.
Discussion
This study showed the distribution of microbial species causing UTIs in the north of Jordan 2003-2005 and their patterns of susceptibility to the most commonly used antimicrobial agents. To help characterise the evolution of antimicrobial resistance in bacterial urine isolates since antimicrobial agents were first widely used at KAUH, existing strain collections of bacterial urine isolates were tested for their susceptibility to a common panel of antimicrobial agents during 2003 to 2005.
As expected, the most common pathogen was E. Coli, followed by Klebsiella pneumonia, Pseudomonas aeruginosa, Enterococcus faecalis and Proteus mirabilis. Generally, this study data is in line with many previous studies (Huang et al., 2014; Sharan et al., 2013; Wang et al., 2014), including the North American Urinary Tract Infection Collaborative Alliance (NAUTICA) study. In the NAUTICA study, forty-one medical centres (30 from the USA and 11 from Canada) participated. The most common organisms found were Escherichia coli (57.5%), Klebsiella pneumonia (12.4%), Enterococcus spp. (6.6%), Proteus mirabilis (5.4%), and Pseudomonas Aeruginosa (2.9%) (Zhanel et al., 2006). Indeed, the NAUTICA study has shown a similarity to the Jordanian bacterial urinary isolates in the most common organisms causing UTI.
According to recent studies around the world, many antimicrobial resistances have been reported in E. coli urinary isolates (Adam et al., 2013; Sharan et al., 2013). Since E. coli is the main uropathogen, the resistance of many antimicrobial agents to E. coli is an important indicator in whether these antimicrobial agents should still be used empirically. Higher rates of susceptibility to many antimicrobial agents have been reported in the general population with UTI worldwide (Barrett et al., 1999; Beunders, 1994; Gruneberg, 1994; Gupta et al., 1999; Huang et al., 2014; Sharan et al., 2013; Wang et al., 2014; Weber et al., 1997; Winstanley et al., 1997)
The present study of bacterial urine isolates for E. coli confirms that high resistance to trimethoprim/sulfamethoxazole was approaching 60%, but also that this rate (yearly range, 60 to 61%) was unchanged during the 3 years studied, from 2003 to 2005 (Table 2). Indeed, previous documented studies conducted in the United States showed a significant increase in trimethoprim/sulfamethoxazole resistance over the decade preceding 1995, from rates of 10% to levels of approximately 16% to 20% (Tadesse et al., 2012).
It has been suggested that fluoroquinolones are a logical choice for empirical therapy of UTIs (Hwang et al., 2014). The data presented in this study appear to validate this assumption; however, the widespread subsequent overuse of fluoroquinolones for such a common infection raises concerns regarding the possibility for the rapid development of resistance (Hwang et al., 2014). This study also noted ciprofloxacin, trimethoprim/sulfamethoxazole, tetracycline and ampicillin demonstrated more than 50% resistance to E. coli from 2003 to 2005 (Table 1). However, the rate of resistance in this study for E. coli to nitrofurantoin (yearly range, 6 to 9%) remained remarkably low from 2003 to 2005. This data regarding the nitrofuration is in line with rates of resistance similar to those published in earlier studies (Schaeffer, 2003). Nitrofurantoin has multiple mechanisms of action (McOsker et al., 1994). This appears to have enabled it to maintain a strong activity against E. coli, despite its use for around 50 years. The in vitro activities of nitrofurantoin found in the present study suggest that they would provide appropriate alternative treatment where trimethoprim/sulfamethoxazole and ciprofloxacin use is no longer wise because of high (more than 50%) rates of resistance.
Generally, all strains were resistant to ampicillin (Table 2). Thus, the use of ampicillin as an empirical treatment for a suspected UTI would not cover the majority of pathogens. Moreover, there were also high rates of resistance to alternative agents such as trimethoprim-sulfamethoxazole which is frequently used in Jordan.
In the literature, there is a general similarity with the rates of resistance to nitrofurantoin, trimethoprim/sulfamethoxazole and amipcillin (Prakash et al., 2013; Sharan et al., 2013). However, there are high rates of resistance to amoxicillin/clavulanic acid and ciprofloxacin and gentamicin compared to other studies such as the ECO·SENS study and Canadian National Surveillance Study (Kahlmeter, 2003). The trend of increasing urinary pathogens resistance during the past few years is similar to our findings (Prakash et al., 2013; Sharan et al., 2013). However, the overall resistance of uropathogens was higher in this study. This disparity may be attributed to differences in the study samples. In addition, laboratory studies may include specimens from asymptomatic patients or patients with external contamination. Local monitoring of resistance, performing of surveillance studies, wise prescribing, and the establishment and use of infection control guidelines are vital to monitor and reduce the possibility of resistance development.
The expected antimicrobial resistance patterns must be considered when selecting empirical antimicrobial treatment strategies for UTI. Consequently, antimicrobial policy should be formulated according to local surveillance data. In our region, the spectrum and antimicrobial resistance patterns of uropathogens were analysed and there was an increase in the rates of resistance to commonly used antimicrobial agents.
In conclusion, the results presented in this study indicate that it is time to reconsider the empirical use of quinolones and to develop clear strategies to counteract the development of further resistance. Since antimicrobial resistance patterns may vary in different regions, it is mandatory that hospitals formulate their antimicrobial policy according to their local resistance pattern, which must be assessed in hospital and laboratory-based surveillance studies. In summary, the data in this study provide much needed information on the prevalence of antimicrobial resistance among pathogens that caused UTI in Jordan. Generally, E. coli is the main bacterial pathogen for UTI and nitrofuratoin demonstrated in vitro the most sensitive antimicrobial agents tested, hence highlighting its great usefulness as a choice for empirical therapy of lower UTI in Jordan.
References
- Adam, HJ, Baxter, MR, Davidson, RJ, Rubinstein, E, Fanella, S, Karlowsky, JA, Lagace-Wiens, PR, Hoban, DJ, Zhanel, GG (2013) Comparison of pathogens and their antimicrobial resistance patterns in paediatric, adult and elderly patients in Canadian hospitals. J Antimicrob Chemother 68 Suppl 1: i31-37.
- Barrett, SP, Savage, MA, Rebec, MP, Guyot, A, Andrews, N, Shrimpton, SB (1999) Antibiotic sensitivity of bacteria associated with community-acquired urinary tract infection in Britain. J Antimicrob Chemother 44(3): 359-365.
- Beunders, AJ (1994) Development of antibacterial resistance: the Dutch experience. J Antimicrob Chemother 33 Suppl A: 17-22.
- Farrell, DJ, Morrissey, I, De Rubeis, D, Robbins, M, Felmingham, D (2003) A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect 46(2): 94-100.
- Filiatrault, L, McKay, RM, Patrick, DM, Roscoe, DL, Quan, G, Brubacher, J, Collins, KM (2012) Antibiotic resistance in isolates recovered from women with community-acquired urinary tract infections presenting to a tertiary care emergency department. CJEM 14(5): 295-305.
- Gruneberg, RN (1994) Changes in urinary pathogens and their antibiotic sensitivities, 1971-1992. J Antimicrob Chemother 33 Suppl A: 1-8.
- Gupta, K, Scholes, D, Stamm, WE (1999) Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281(8): 736-738.
- Haller, M, Brandis, M, Berner, R (2004) Antibiotic resistance of urinary tract pathogens and rationale for empirical intravenous therapy. Pediatr Nephrol 19(9): 982-986.
- Huang, LF, Lo, YC, Su, LH, Chang, CL (2014) Antimicrobial susceptibility patterns among Escherichia coli urinary isolates from community-onset health care-associated urinary tract infection. J Formos Med Assoc.
- Hwang, TJ, Hooper, DC (2014) Association between fluoroquinolone resistance and resistance to other antimicrobial agents among Escherichia coli urinary isolates in the outpatient setting: a national cross-sectional study. J Antimicrob Chemother.
- Kahlmeter, G (2003) Prevalence and antimicrobial susceptibility of pathogens in uncomplicated cystitis in Europe. The ECO.SENS study. Int J Antimicrob Agents 22 Suppl 2: 49-52.
- Karaca, Y, Coplu, N, Gozalan, A, Oncul, O, Citil, BE, Esen, B (2005) Co-trimoxazole and quinolone resistance in Escherichia coli isolated from urinary tract infections over the last 10 years. Int J Antimicrob Agents 26(1): 75-77.
- Kiffer, CR, Camargo, EC, Shimakura, SE, Ribeiro, PJ, Jr., Bailey, TC, Pignatari, AC, Monteiro, AM (2011) A spatial approach for the epidemiology of antibiotic use and resistance in community-based studies: the emergence of urban clusters of Escherichia coli quinolone resistance in Sao Paulo, Brasil. Int J Health Geogr 10:
- Kurutepe, S, Surucuoglu, S, Sezgin, C, Gazi, H, Gulay, M, Ozbakkaloglu, B (2005) Increasing antimicrobial resistance in Escherichia coli isolates from community-acquired urinary tract infections during 1998-2003 in Manisa, Turkey. Jpn J Infect Dis 58(3): 159-161.
- McOsker, CC, Fitzpatrick, PM (1994) Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother 33 Suppl A: 23-30.
- Prakash, D, Saxena, RS (2013) Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of meerut city, India. ISRN Microbiol 2013:
- Schaeffer, AJ (2003) Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. J Urol 170(1): 335-336.
- Shaifali, I, Gupta, U, Mahmood, SE, Ahmed, J (2012) Antibiotic susceptibility patterns of urinary pathogens in female outpatients. N Am J Med Sci 4(4): 163-169.
- Sharan, R, Kumar, D, Mukherjee, B (2013) Bacteriology and antibiotic resistance pattern in community acquired urinary tract infection. Indian Pediatr 50(7):
- Sire, JM, Nabeth, P, Perrier-Gros-Claude, JD, Bahsoun, I, Siby, T, Macondo, EA, Gaye-Diallo, A, Guyomard, S, Seck, A, Breurec, S, Garin, B (2007) Antimicrobial resistance in outpatient Escherichia coli urinary isolates in Dakar, Senegal. J Infect Dev Ctries 1(3): 263-268.
- Tadesse, DA, Zhao, S, Tong, E, Ayers, S, Singh, A, Bartholomew, MJ, McDermott, PF (2012) Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950-2002. Emerg Infect Dis 18(5): 741-749.
- Usluer, G, Ozgunes, I, Leblebicioglu, H (2005) A multicenter point-prevalence study: antimicrobial prescription frequencies in hospitalized patients in Turkey. Ann Clin Microbiol Antimicrob 4:
- Wang, Q, Zhang, X, Xia, P, Chen, Y, Feng, W, Sun, F (2014) Spectrum and antimicrobial resistance of uropathogens from patients with urinary tract infection in urology and non-urology departments. Saudi Med J 35(2): 198-200.
- Weber, G, Riesenberg, K, Schlaeffer, F, Peled, N, Borer, A, Yagupsky, P (1997) Changing trends in frequency and antimicrobial resistance of urinary pathogens in outpatient clinics and a hospital in Southern Israel, 1991-1995. Eur J Clin Microbiol Infect Dis 16(11): 834-838.
- Winstanley, TG, Limb, DI, Eggington, R, Hancock, F (1997) A 10 year survey of the antimicrobial susceptibility of urinary tract isolates in the UK: the Microbe Base project. J Antimicrob Chemother 40(4): 591-594.
- Wise, R, Hart, T, Cars, O, Streulens, M, Helmuth, R, Huovinen, P, Sprenger, M (1998) Antimicrobial resistance. Is a major threat to public health. BMJ 317(7159): 609-610.
- Zhanel, GG, Hisanaga, TL, Laing, NM, DeCorby, MR, Nichol, KA, Weshnoweski, B, Johnson, J, Noreddin, A, Low, DE, Karlowsky, JA, Hoban, DJ (2006) Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 27(6): 468-475.








