R. Banumathi1*, M. Padmaj1 and M. Deecaraman2
1Unit of Invertebrate Reproduction and Pharmacological Endocrinology, Department of Zoology, Sir Theagaraya College, Chennai - 21, India.
2M.G.R University, Maduravoyal, India.
Corresponding Authors E-mail: banu3235@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/434
Abstract
The present study describes the morphology and histological functions of the testes and vas deferens of Spiralotelphusa hydrodroma. The morphohistological of this fresh water crab is presented, with a detailed analysis of the histology of the regional structure of the testis and vas deferens. The H-shaped testis is subdivided into three distinct parts: anterior, iintermediate and posterior. The vas deferens is continuous with the posterior part of the testis, ends at the ejaculatory duct , and is subdivided into three distinct regions: proximal, medial and distal. The anterior part of the testis exhibits convoluted lobules including seminiferous tubules. Whereas the intermediate part of the testis has a simple columnar epithelium, the posterior part of the testis has a simple squamous epithelium. In the proximal and medial regions of the vas deferens, there is a simple cubic epithelium, while in the distal region of the vas deferens there is a high columnar epithelium. Spermatophores are first formed in the convoluted proximal region of the vasdeferens and are encapsulated by mainly protein secretions in the distal region. These results suggest that the testis and vas deferens of Spiralotelphusa hydrodroma show similarity to the testis and vasdeferens of other brachyuran crabs except for some slight differences. Spermatophores were observed in both vasdeferens regions suggesting that they participated in the maturation of the spermatozoids preparing the male functionally for fertilization.
Keywords
Spiralotelphusa hydrodroma; Cubical epithelium; Germinal zone; Vasdeferens; Spermatids; spermatophores.
Download this article as:| Copy the following to cite this article: Banumathi R, Padmaja M, Deecaraman M. Study on the Morphohistological Patterns and Functions of Vasdeferens and Spermatophores in Male Fresh Water Crab Spiralothelphusa hydrodroma. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Banumathi R, Padmaja M, Deecaraman M. Study on the Morphohistological Patterns and Functions of Vasdeferens and Spermatophores in Male Fresh Water Crab Spiralothelphusa hydrodroma. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2782 |
Introduction
In many decapod crustaceans, the male produces discrete aggregations of spermatozoa embedded in some form of protective covering, termed the spermatophores. merely deposited on the sternum of the females. In this way, the spermatozoa can be retained in a viable state by the female until such time the ova are ready for fertilization. In Malacostraca, spermatophores assume a variety of shapes, and show variation in their chemical composition..A correlation between the spermatophore morphology and the type of fertilization has been suggested by Spalding, J.F. (1942). and Subramoniam, T. (1982). for Crustacea. The male reproductive organs of the malacostracans, including the decapods, are in the cephalothorax or thorax (Krol et al., 1992). In decapods, the male reproductive tract consists of paired testes and genital ducts. Each genital duct consists of a collecting tube, a vas deferens with regionally distinct functions, and an ejaculatory duct ending in a seminal vesicle or in a terminal ampoule, depending on the species (Krol et al., 1992). When spermatogenesis is complete, the sexual cells are transported to the vas deferens (Hinsh and Walker, 1974). The vas deferens is a tubular duct (Krol et al., 1992) in which the sperm cells become encapsulated forming spermatophores (Hinsch and Mcknight, 1988; Sainte-Marie and Sainte-Marie, 1999; Spalding, 1942). Although much has been described on testis and vas deferens morphology, only few studies have dealt with crabs Mohamed, K.S., Diwan. A.D. (1993).reported on the spermatogenesis and spermatophore formation in Penaus indicus an Indian white prawn. They suggested that the spermatophore formation took place in the mid vasdeferens. The latter include light microscopy studies of the species Menippe mercenaria (Binford, 1913), Pachygrapsus marmoratus (Mouchet, 1931), Callinectes sapidus (Cronin, 1947; Johnson, 1980), Portunus sanguinolentus (Ryan, 1967), Libinia emarginata(Hinsh and Walker, 1974: Testis and vas deferens morphology has been described for several other species of crustaceans, including Panulirus (Talbot and Summers, 1978), Thenus orientalis (Burton, 1995), Spermatophore formation has been studied for a number of crustaceans using light microscopy, including the crab species Carcinus. sapidus (Cronin, 1947; Johnson, 1980), Scylla serrata (Uma and Subramoniam, 1979), Carcinus maenas (Spalding, 1942), The present study of the testis and vas deferens morphology and spermatophore formation of the Spiralotelphusa hydrodroma,was selected , in order to understand the reproductive process of this economically important species dwelling in rice and paddy fields as noted to be important area of crustacean decapods and this particular species which is used as edible one has enriched nutritive value. The objective of this study is to characterize the morphology of the reproductive system of male species including the testes, vasdeferentia and spermatophore formation,from both macroscopic and microscopic points of view, and to know more about spermatophore formation in the vasdeferens , and the spermatozoans present, seems to be prepared for successful fertilization.
Material and Methods
The live specimens of Spiralothelphusa hydrodroma were collected from a nearby paddy fields and also from the water bodies, of then keeranur pond situated at vilambar village, of villipuram district ,Tamilnadu, during the months between October 2009 to November 2010 . This species is commonly found mostly everywhere in all the areas of paddy fields. After removal of the carapace, both the testes and vasdeferens were dissected and fixed in Bouin’s solution for 4 hrs. For the histological study ,testis, proximal vasdeferens, mid vasdeferens, distal vasdeferens, ejaculatory duct consisting of spermatophric cord of male Spiralotelphusa hydrodroma were dissected.,and fixed in Bouins fluid. For obtaining good sections tissues were fixed in Ciaccious fluid (Subramoniam, 1982). After fixation for 24 hrs the tissues were dehydrated in different alcoholic series and embedded in paraffin wax (melting point 52°C – 54°C). The paraffin blocks were sectioned at 4-6 μm, affixed to albuminised slides, deparaffinised in xylol and stained in Ehrlichs haematoxylin and counterstained in aqueous eosin. The stained sections were latter cleared in xylol and mounted in D.P.X (Bancroft and Stevens, 1977, Humason, 1979) , Binford (1913) using histological sections studied the spermatogenesis in Carcinus maenas.. Bawab, S.R.A. (1974).studied on the spermatophore formations in Penaeus kerathurus.Burton, T.E. (1995).studied the spermatid pathway and associated reproductive structures of the squat lobster Thenus orientalis (Lund).and to.determine the morphology of spermatophores . Once the male crab has attained sexual maturity the gonoductal ways seems to be fully charged with a milky seminal fluid which contains large numbers of spermatophores and also droplets of a clear fluid. This observation agrees with the statement of Broekhuysen, G.J. (1936). who remarks: ‘the state of swollenness and the whitish colour of the reproductive organs vary, it is true, a little, but there is strong evidence that practically all animals, as regards the development of their sexual organs, could copulate all the year round.’ No relationship could be found between the moulting cycle and the state of the gonads in male crabs. The testes and vas deferentia might be equally mature, both in macroscopic and microscopic appearance, in freshly moulted crabs with completely soft shells, as in those with a thick, red, barnacle-encrusted shell which had obviously not moulted for a long time. It is not easy in dissection to say where the testis ends and the vas deferens begins, but microscopical examination reveals a point where the cells round the central lumen begin to form a simple cubical epithelium. Taking this region as the beginning of the vasdeferens it is then possible to divide the remains of the coiled tube into four distinct functional regions as shown in the( Fig 1&2) which, however,tend to merge into one another in general histological detail. Nearest to the testis, a region where sperms occur free in the lumen of the duct unaccompanied by any fluid having a characteristic staining reaction.to be seen, together with fully formed spermatophores.
 |
Figure 1: Photomicrograph showing the entire animal of male and female crab Spiralotelphusa hydrodrma |
Results
Most crustaceans have separate sexes, as in the other decapod crustaceans, though sexual dimorphism is less marked than in others, sex can be distinguished externally by the following difference.The males have a relatively narrow abdomen while that of females are broad and truncate. and blunt in females.(Fig-1&2 ). The claws of the adult males are larger than those of females. The antennules of males are more abundantly supplied with olfactory hairs than those of females. But the most sure point of identification of sexual differences is the location of male genital openings on the bases of the fifth pair of walking legs attached to the last thoracic segment.(Fig-2) The sexual dimorphism studied according to Adiyodi, K.G. and Adiyodi, R.G. (1974).
 |
Figure 2: Photomicrograph showing the ventral view of male crab Spiralotelphusa hydrodroma |
The male reproductive system
The reproductive system of male crustaceans consists of the paired testes, vasdeferens, seminal vesicles and a genital aperture .The testes are white, elongated structures located near the pericardial area. The testicular morphology is similar to the ovarian morphology in that the testis also maintains an H-like appearance. From each testis emerges a vas deferens that links it to the exterior by the gonophores, located on the base of the fifth pair of pereiopods. The vasdeferens has three microscopically distinct regions that contain spermatophores in different stages of maturation. Spermatogenesis is mostly characterized by the differentiation of sperm cells, and their maintenance before fertilization. ,The testis contains 10–15 lobes each composed of many seminiferous ubules, whose shape changes according to the stage of spermatogenesis(Fig-3)
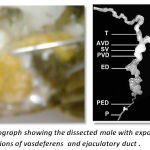 |
Figure 3: Photograph showing the dissected male with exposed testis and regions of vasdeferens and ejaculatory duct |
Histology of mature testis
According to Pearson (1908) the testes are small paired tubular organs lying laterally in the anterior part of the body cavity and closely applied to the hepatopancreas.in Carcinus maenus. Structurally they are almost identical and composed of closely packed follicles in which the germ cells may be seen in different stages of development. Each follicle is surrounded by a thin epithelial wall. An unripe follicle shows a densely packed mass of developing cells with no central lumen, while a ripe follicle shows a central cavity in which the ripe spermatozoa lie free. The sperms closely resemble those of the crab, Cancer magister, as described by Fasten (1918).The development of what he terms the ‘ nuclear-mitochondrial cup’ makes the sperms very much larger than- the spermatids from which they have developed. The unripe sex cells, spermatogonia and spermatids, stain red.The cross section of mature testis in Spiralotelphusa hydrodroma reveals distinct connective, muscular and epithelial linings measuring 3 μm, 4μm and 5 μm in thickness in diameter. These layers stains well from pink to red colour in haemotoxylin and eosin. In the mature male many seminiferous tubules are filled with numerous spermatogonial cells displaying different stages of maturation. The spermatogonial cells stain darkly in the haemotoxylin and eosin and the lumen measures 10 μm in diameter and consists of large number of spermatocytes, spermatids and spermatozoa which stain darkly in haemotoxylin and eosin. In addition to the spermatozoa the sperm mass (aggregation of spermatozoa) with moderate amount of secretory substances staining light pink in haemotoxylin and eosin (Fig-4) are also seen.
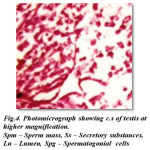 |
Figure 4: Photomicrograph showing c.s of testis at higher magnification. Spm – Sperm mass, Ss – Secretory substances, Lu – Lumen, Spg – Spermatogonial cells |
Morphology of vasdeferens in male crab
Each multilobed testis connects to a long tube, the proximal vas deferens, leading into the Ushaped medial vasdeferens (Bauer & Cash, 1991). Depending on morphological and histological characters the vasdeferens of mature male is orbitorialy divided into three regions (For the convenience of study)The proximal vasdeferens starts from the mid region of the testis. It is very short, white cylindrical, translucent, thin, and loosely coiled duct. It is difficult to uncoil, on which it may get disintegrated if handled roughly . The mid vasdeferens is the continuation of the proximal vasdeferens. It is slight thick and opaque and massive white tube, and is loosely coiled. The distal vasdeferens can easily be distinguished as it shows slight thickness and long in its length and milky white in colour.Tlast terminal portion is the ejaculatory duct known for the storage of spermatophores
Histology of Proximal, Middle and Distal vasdeferens in the mature male crab
Proximal vasdeferens
The cross section of proximal vasdeferens stains well in haemotoxylin and eosin. The outerlayer connective stains light pink and the middle musuclar stains darkly in colour and measures about 2 μm and 3μm in diameter respectively. The inner epithelial layer measures 5μm in diameter and the epithelial layer stains light violet in colour. This is enriched with secretory granules. The lumen measures about 25μm in diameter and stains light pink and contains free sperms, sperm mass and the spermatophores. The spermatophores are in turn is packed with sperms. The condensation of the matrix towards the formation of the spermatophoric layer is clearly seen. These spermatophores are found mixed with a gelatinous substance (Fig- 5). The formation of typhlosole can be seen in the proximal vasdeferens.
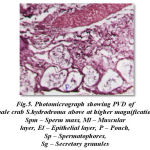 |
Figure 5: Photomicrograph showing PVD of male crab S.hydrodroma above at higher magnification Spm – Sperm mass, Ml – Muscular layer, El – Epithelial layer, P – Pouch, Sp – Spermatophores, Sg – Secretory granules |
Mid vasdeferens
The cross sections of mid vasdeferens in the mature male does not show any marked difference except in the improvement in lumen width and thickenss in muscular layer. The outer connective layer measures 3 μm in thickness. The size of lumen vary at different position in the mid vasdeferens. The mid vasdeferens stains dark red in colour in haemotoxylin and eosin. The muscular layer is thicker measuring 8 μm diameter and the epithelial layer measuring 5 μm in thickness. The staining reactions of various layers is similar as in proximal vasdeferens except the inner matrix shows a deep red colour stain in haemotoxylin and eosin and can be easily distingusihed from the outer layer of matrix which is close to the epithelial layer lining.The lumen is more spaceous and measures 32 μm in diameter. Many spermatophores are found in the lumen. The mid vasdeferens shows a bifid structure of typhlosole which shows a foliaceous appearnce. The wall of the typhlosole also stains darker and contains secretory granules (Fig- 6).
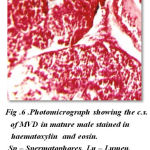 |
Figure 6: Photomicrograph showing the c.s. of MVD in mature male stained in haematoxylin and eosin. Sp – Spermatophores, Lu – Lumen, Ss – Secretory substances |
Distal vasdeferens
The cross section of distal vasdeferens showing different regions reveal interesting features. The cross sections show extremely thick muscular layer measuring 12 μm in thickness and stains dark deep red colour in haemoloxylin and eosin and thin epithelial layer measures 1 to 2μm thickness and stains light pink in colour. The lumen appears irregular in shape and forms many pouches which are more in number with more alveoli like pockets or loops. This stains dark pink in colour and measures about 42 μm in diameter. The spermatophores are well seen within these pouches and deeply stains in haemotoxylin and eosin. These pouches show many vacuolar spaces having central nuclei. The typhlosole stains light pink in colour and appears to be more simplified which gradually reduce ultimately and disappear at the hind end of the posterior distal vasdeferens. Each region of the distal vasdeferes shows many variation with regard to different events occurring in this region.The DVD of regions shows many pouches filled with spermatophores. The other regions also show the lumen filled with secretory substances and secretory granules and aggregation of spermatophoric cord with more number of spermatophores. Similarly the regions shows the secretory substances and the reducing of typhlosole and also exhibits the presence of numerous number of spermatophores and the lumen is filled with more sperm mass contents and lodged with secretory substances. The region of distal vasdeferens shows some empty pouches exhibiting the absence of spermatophores, sperm mass and secretory substances, which is aggregated into a gelatinous matrix and convolutes to form the spermatophoric cord which later ejects towards the ejaculatory duct.(Fig-7)
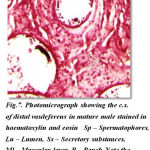 |
Figure 7: Photomicrograph showing the c.s. of distal vasdeferens in mature male stained in haematoxylin and eosin Sp – Spermatophores, Lu – Lumen, Ss – Secretory substances, Ml – Muscular layer P – Pouch Note the pouches filled with spermatophores |
Morphology of ejaculatory duct
The ejaculatory duct is the terminal end portion of distal vasdeferens. In immature it is thin narrow, muscular white tube ending inside the posterior end of fifth walking and opens outside as genital aperture. The ejaculatory duct is very clear in the mature males.(Fig -8). It is broad, more whitish in colour and seems to be highly muscular tube ending towards the fifth walking leg and open outside as genital aperture, appearing to be more prominent in its appearance.
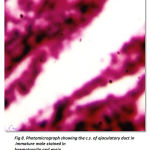 |
Figure 8: Photomicrograph showing the c.s. of ejaculatory duct in immature male stained in haematoxylin and eosin. Ss – Secretory substances, Sg – Secretory granules. |
Histology of ejaculatory duct
The cross section of ejaculatory duct in the mature male shows, an outer connective layer measuring 6 μm thickness and stains light brown in haemotoxylin and eosin. On one side of the wall is surrounded by thin epithelial layer measuring 2 μm thickness and stains lighter. The epithelial cells are elongated, and uniformly thin in shape and are highly secretory. The muscular layer measures 15μm in thickness stains intensely. The lumen shows darkly stained secretory spherical granules,and sperm mass..(Fig -9)
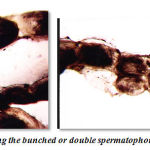 |
Figure 9: Photograph showing the bunched or double spermatophoric cord. |
Nature of gelatinious spermatophoretic cord
Terminal section of the male reproductive tract is of course the ejaculatory duct which is characterised by a very narrow crescentic lumen caused by the occurrence of a muscular prominence from the wall of ejaculatory duct which is highly muscular. This leaves an impression that the spermatophoric cord might be squeezed out in a single file to be deposited in the sternum of the females.(Fig10) Spermatophore formation has been studied in two brachyurans, Ocypode platyarsis (Sukumaran, 1985; and Parathelphusa hydrodromous (Mathews, 1953); In Ocypode platyarsis, sperms released from the testicular acini flock together randomly in the upper reaches to the anterior vasdeferens (AVD).In Libina emarginata and Libina dubia, the spermatophores are formed in the anterior vasdeferens itself. The spermatozoa are first surrounded by a dense material and by a less dense material which together eventually form the spermatophoric wall (Hinsch and Walker, 1974). The spermatophoric material in crabs of the genus Carcinous maneous is secreted by epithelial cells distributed at the anterior end of the vasdeferens where several of the spermatozoa are enclosed in condensed secretory material to form a spermatophore (Langreth, 1969).Among natantian decapods, in Penaeus indicus (Muthuraman, 1986), the spermatophore is formed outside the body of the male, while in Macrobrachium idella (Sreekumar, et al., 1982) the spermatophore appears to be formed in the seminal vesicle itself..
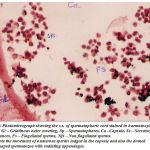 |
Figure 10: Photomicrograph showing the c.s. of spermatophoric cord stained in haematoxylin and eosin. Gl – Gelatinous outer covering, Sp – Spermatophores, Ca –Capsule, Ss – Secretory Substances, Fs – Flagellated sperms, Nfs – Non flagellated sperms. Note the movement of numerous sperms lodged in the capsule and also the domed shaped spermatozoa with radiating appendages. |
Histology of spermatophoric cord
The cross section of spermatophoric cord shows numerous sperms lodged in a capsule or sac. The lumen or the space of each spermatophoric capsule shows many non flagellated free grouped sperms stained darkly ( Fig11). Obviously S.hydrodroma have spermatophoric cord strategy and thereby revealing an independent line of progress with regard to sperm transfer. In order to confirm the validity, the testes and the reproductive tract of S.hydrodroma at carapace length of 3.5 cms and 5.5 cms are histologically examined and it has been found that in the case of former the testes possess mature spermatozoa but the reproductive tract contains only a few spermatophores. On the contrary in the latter case of specimens having the carapace length of 6.5 cms and above possess not only large number of fully formed mature spermatozoans in the testes but also fully formed spermatophores plenty in the reproductive tract. Thus in the present study could be comparable to the physiological and functional mature conditions of other crabs., Spermatophores are comprised of (1) spermatozoa which are usually supported in a matrix (ground substance) of testicular (in some cases of vasdeferens) origin, and (2) one to several layers of a cellular secretions, produced by the vasdeferens, that surround the spermatozoa. The spermatophoric wall serves to protect the spermatozoa during transfer to the female and during storage function in spermatheca. Calman (1909) classified the repantantian spermatophores into pedunculated and non-pedunculated (tubular) types based on morphology and taxonomic value.Most anomurans hermit crabs have pedunculated spermatophores;But in this present study the sperm cord covered with the sheath is fragmented into separated spermatophore each of which attaches itself to a membrane by a stalk peduncle. Spermatophore are generally ovoid to spherical and in each spermatozoa show a dome shaped structure with a small radiating processes (Fig -11).The strategy of sperm transfer in various decapod crustaceans appear to be essentially the same as the spermatophore which indeed reflect their phylogeny.
Discussion
The observations on the morphology of the male reproductive system of S. hydrodroma suggest a basic similarity to that of other decapods especially the macrurans. As in the case of the prawn, Panaeus monodon Subramanhyam, M. and Ganapathi, P.N. (1975) .spiny lobster, Panulirus homarus Radha, T. (1983) and the Hawaiian red lobster, Enoplometopus occidentalis ,The male reproductive system consists of a `H’ shaped testes and the vasdeferens emerging from the outer margin of the post-commissural limb of each testes. In almost all the macrurans the precommissural limbs of the testes are shorter than the post-commissural limbs. Again the vasdeferens of these animals appear to be similar except with minor differences of male reproductive system is concerned. Needless to point out that even the branchyuran crabs, Portunus sanguinolentus Ryan, E.P. (1967b). and Cardisoma carnifex Kalyanaram and Chandran, M.R. (1980) are not very different from the macruran, as both of them belong to the same sub order Reptantia of the order Decapoda. Even the anomuran mole crab, Emerita asiatica Sukumaran, M. (1985). has the same basic `H’ pattern even though the pre-commissural limbs are longer as in the case of brachyura. In addition, the testes at the posterior end continue as a vasdeferens unlike in the case of S.hydrodroma in which vasdeferens starts at the outer margin of the post-commissural limb of the testes leaving a long portion of the testes behind the junction of testes and vasdeferens..The present observation on the Spiralotelphusa hydrodroma obviously confirms to the macruran pattern. An entirely different pattern is obvious in the stomatopod S. holoschista in which the vasdeferens is the continuation of the anterior end of the testes.As in the case of brachyuran crabs and stomatopod Squilla holoschista, and in the vasdeferens has three morphologically distinguishable regions, viz., PVD, MVD,and DVD whereas Radha, T. (1983). reports that there are only two morphologically distinguishable regions in the spiny lobster, Panulirus homarus eventhough it is a macruran Haley, S.R. (1984 )also agrees with this division with reference to E. occidentalis. The anomurans also show only two divisions namely proximal and distal regions Sukumaran, M. (1985).The T.orientalis shows a similarity to the brachyurans rather than the anomurans..
In this particular investigations on Spiralotelphusa hydrodroma morphologically four regions were found and the last posterior end called ejaculatory consists of the gelatinious cord like called spermatophoric cord which carries spermatophores marks intrest to this topic .
Histological investigations carried out on the four sections of vasdeferens., PVD, MVD DVD, and EJD ,distinguished morphologically on the basis of features like appearance, colour, width, nature of coarse such as coiling etc., could confirm these regional differentiation. Eventhough, the entire reproductive tract contains a wall made up of only the same three layers namely outer connective middle muscular and inner epithelial tissue, the differential growth revealed by thickness, in foldings, modification of the inner epithelial layer into glandular secretory cells etc., have led to clearcut regional specialisation.The cross section in MVD. in the male reveals an outer connective, middle muscular and inner epithelial layers which are very thin. The lumen is filled with a fluid which appears to be dark in staining and are loaded with secretory granules. Whereas, the mature MVD. shows a marked improvement in the lumen width and thickness of muscular layer and stains darker in hematoxylin and eosin. The lumen shows more spaceous appearances filled with more number of spermatophores and reveal a bifid structure with foliaceous appearance. This seems to be a typhlosole which contains secretory particles.In S. holoschista the a MVD. is believed to be storage in function Deecaraman, M. (1977)However Radha, T. (1983). and Haley, S.R. (1984). have not reported the occurrence of a midvasdeferens in the spiny lobster P. homarus and in Hawaiian red lobster, E. occidentalis respectively as they have recognised only two regions in the male reproductive tract.The third and the last region of the reproductive tract is the DVD and a terminal ejaculatory duct (ED). This division is more obviously seen in the histological preparations. The DVD is closer in nature to the MVD. in having pouches which not only increase in number but also in complexity developing further into loops. However, the muscular layer has increased in thickness with a corresponding decrease in the epithelial layer suggesting the fact that they are no more involved in the secretory activity. Not withstanding this reduction in the size of the epithelial layer, the loops and pouches of DVD contain long but narrow epithelial cells which appear to be secretory. The lumen appears to the more irregular in shapes and the pouches show many vacuolar spaces filled with many spermatophores deeply stained. Thus, this portion of the DVD has a double function such as storage and conduction while at the same time adding some more secretions to mix the spermatophores. Though such a secretion of the tract with similar function has not been described in the spiny lobster Radha, 1983). Ryan (1967b) in the brachyuran, P. sanguinolentus describes a corresponding section with almost a similar function.The functions attributed in the present study to the DVD, however, cannot be compared to those of C. carnifex as in this crab individual spermatophores are formed in the PVD only to be dissolved in the terminal DVD and to aid in this function, the animal has the accessory gland. Nevertheless, the DVD of C. carnifex is supposed to have a storage function.The cross section of spermatophoric cord shows numerous sperms lodged in a capsule or sac. The lumen or the space of each spermatophoric capsule shows many non flagellated free grouped sperms stained darkly.(Fig-10 ).
Each spermatozoa show a dome shaped structure with a small radiating processes.The strategy of sperm transfer in various decapod crustaceans appear to be essentially the same as the spermatophore which indeed reflect their phylogeny. Obviously the stomatopod have adopted different method namely the spermcord strategy which again reflect the decapods. Within the decapods the spermatophore are formed of two types as pedunculated and non pedunculate which may be thought a side line of evolutionary progress evolving from spermatophore..
Conclusion
These results suggest that the testis and vas deferens of Spiralotelphusa hydrodroma show similarity to the testis and vasdeferens of other brachyuran crabs except for some slight differences. Spermatophores were observed in both vasdeferens regions suggesting that they participated in the maturation of the spermatozoids preparing the male functionally for fertilization.
References
- Adiyodi, K.G. and Adiyodi, R.G. (1974). Comparative physiology of reproduction in arthropods. Adv. Comp.Physiol., Biochem; 5 : 37 – 84.
- Bawab, S.R.A. (1974). The formation of the spermatophore in Penaeus Kerathurus (Forskal, 1775) (Decopda : penaeidea). 1. The initial formation of a sperm mass.Crustaceana, 26 : 273 – 285
- Broekhuysen, G. J., 1936.—”Development, Growth and Distribution of Caroinides maenas”, ‘Arch, neerl. Zool.’, 2
- Broekhuysen, G.J. (1936). On development, growth and distribution of Carcinus maenas (L). Archs. neorl. Zool., 2 : 257 – 399.
- Calman, W.T. (1909). Crustacea. A. Treatise on Zoology, part 8 – A and C Black, London.A field study J. Crust. Biol., 12 (4) : 655 – 660.
- Deecaraman, M. (1977). Studies on the male reproductive tract and accessory organs of stomatopoda, M.Phil Dissertation. University of Madras.
- Fasten, N. (1918). Spermatogenesis of the Pacific Coast edible crab, Cancer magister Dana. Biol. Bull., 34 : 277- 306.
- Fasten, N. (1926). Spermatogenesis of the black clawed crab, Lophopanopeus bellus (Stimpson) Rathbun. Ibid., 50(4) : 277 – 292.
- Haley, S.R. (1984). Spermatogeneis and spermatophore production in the Hawaiian red lobster, Enoplometopus occidentalis (Randall) (Crustacea, Nephropidae).J. Morphol. 180 : 181 – 193.
- Hinsch, G.W. (1970). Some factors controlling reproduction in the spider crab., Libinia emarginata, Biol. Bull. 139 : 4 – 10..
- Kalyanaram and Chandran, M.R. (1980). A note on the occurrence of a male accessory sex glands in the land crab, Cardiosoma carnifex (Herbst). Pro. 1. All Ind. Sym. Inv. Reprod.
- Madras University, 61 – 67.
- Mathews. D.C. (1953a). The origin of the spermatophoric mass of the sand crab, Hippa pacifica, Q. J. Micros. Sci., 97 : 257 – 268.
- Mohamed, K.S., Diwan. A.D. (1993). Spermatogenesis and spermatophore formation in the Indian white..Mouchet, S. (1931).
- Spermatophores des crustace’s decapodes, Anomures et Brachyoureset castration parasitaire Chez quelques paguers. Ann. Inst. Oceanogr, 6 : 1 – 203.
- Radha, T. (1983). Investigation on the origin and nature of spermatophoric mass in a spiny lobster Panulirus homarus (Linnasus) (Crustacea: Macrura). M.Phi., Dissertation,University of Madras.
- Ryan, E.P. (1967a). Structure and function of the reproductive system of the crab, Portunus sanguinolentus (Herbst) (Brachyura; Portunidae). The female system. Pro. Symp. Crustacean. Mar. Biol. Assoc. India. Ernakulam, Part – 2;522 – 544.
- Ryan, E.P. (1967b). Structure and function of the reproductive system of the crab Portunus sanguinolentus (Herbst). (Brachyura ; Portunidae). The male system, Proc. Symp. Crust. Mar. Biol. Assoc. India. Part – 2 : 506 – 521.
- Spalding, J.F. (1942). The nature and formation of the spermatophore and sperm plug in Carcinus maenus. Q.J. Microsc. Sci., 83 : 399 – 422.
- Subramanhyam, M. and Ganapathi, P.N. (1975). The biology of the prawns, Penaeus monodon fabricius from the Godavari Estuarine system. Bull. Dept. Mar. Sci. Univ. Cochin. VII. 3 : 653 – 670
- Subramoniam. T. (1977). Continuous breeding in the tropical anomuran crab, Emerita asiatica from Madras coast. Adv. inver. repro. Vol.I Adiyodi. K.G. and Adiyodi. R.G. (Ed) Peralan. Kennoth. Karivellur, Kerala.
- Subramoniam, T. (1982). Manual of research methods for marine invertebrate reproduc
- Subramoniam, T. (1984). Spermatophore formation in two intertidal crabs. Albunea simnista and Emerita asiatica (Decapoda: Anomura). Bio. Bull, 166 : 78 – 95.
- Sree kumar, S., Adiyodi, R.G. and Adiyodi, K.G. (1982). “Aspects of semen production in Macrobranchium sp; in giant prawn.








