Kunjumon Dayana1 and Megaravalli R. Manasa2
1Department of Pharmacology, Pushpagiri institute of medical sciences and research centre, Thiruvalla, Kerala, India.
2Department of Pharmacology, Karwar institute of medical sciences, Karwar, Karnataka, India.
Corresponding Author E-mail: dr.manasamr@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1634
Abstract
Lipid peroxidation generates free radicals. These free radicals are scavenged by antioxidant defense mechanisms. An imbalance between the free radicals generation and antioxidant mechanisms can result in tissue damage. Several drugs are known to induce lipid peroxidation which can be responsible for their toxic potential. Hence the current study was planned to assess the effect of ceftriaxone, a third generation cephalosporin, on lipid peroxidation and levels of antioxidants in albino mice. Ceftriaxone was injected intraperitoneally at two doses - 100 mg/kg body weight; 200 mg/kg body weight – to albino mice. TBARS (Thiobarbituric acid reactive substance) levels in plasma, erythrocytes as well as tissue and the antioxidant enzymes activities were estimated. The data from ceftriaxone groups was analyzed with control group using ANOVA and Dunnett’s test as post hoc. Ceftriaxone (100 mg/kg body weight) did not alter TBARS levels compared to control. Ceftriaxone - 200 mg/kg body weight, has significantly increased TBARS levels. The activities of antioxidant enzymes were significantly decreased by ceftriaxone at these doses. The present study demonstrates that ceftriaxone has the potential for lipid peroxidation induction and reduction in the antioxidant enzymes acitivities in albino mice.
Keywords
Antioxidants; Ceftriaxone; Lipid Peroxidation; Mice
Download this article as:| Copy the following to cite this article: Dayana K, Manasa M. R. Evaluation of the Effect of Ceftriaxone on Lipid Peroxidation and Antioxidant Levels in Mice. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Dayana K, Manasa M. R. Evaluation of the Effect of Ceftriaxone on Lipid Peroxidation and Antioxidant Levels in Mice. Biomed Pharmacol J 2019;12(1). Available from: http://biomedpharmajournal.org/?p=25769 |
Introduction
Lipid peroxidation is oxidative damage of polyunsaturated fatty acids mediated by free radicals.1 It is a destructive process and involved in worsening of tissue injuries.2 Free radicals are continuously produced in the body but they are scavenged by antioxidant defense systems that also prevent the oxidatively damaged molecules from getting accumulated.3 The antioxidants such as vitamin E, C and reduced glutathione (GSH) (non enzymes) and superoxide dismutase (SOD), Catalase (CAT), glutathione peroxidase (GPx) (enzymes) have a major role in the defending the body against this damage.4,5,6 However, when the defense systems are overwhelmed as happens in acute oxidative stress, it results in changes in the structural and functional components of cells.7
According to some studies, the pathogenesis of various conditions like radiation damage, ischemia reperfusion injury, atherosclerosis, diabetes mellitus, carcinogenesis and neurodegenerative diseases are by mediation of free radicals.8 Drug induced lipid peroxidation is the basis of toxicities of several drugs such as cardiotoxicity of doxorubicin,9 gastric mucosal injury by indomethacin,10 renal injury by cisplatin,11,12 nephrotoxicity of gentamicin,13,14 etc. Hence current study was conducted for assessing the effect of ceftriaxone on lipid peroxidation and activities of antioxidant enzymes in mice.
Materials and Methods
Chemicals
The ceftriaxone and other biochemical compounds – GSH, NADH, 1,1’,3,3 – tetramethoxy propane were procured from sigma – Aldrich chemicals Pvt. Ltd., Bangalore, India. Heparin, thiobarbituric acid (TBA), trichloro acetic acid, 2, 4 – dinitro phenyl hydrazine, 1 – Choloro -2, 4 – dinitrobenzene, nitroblue – tetrazolium, phenazine methosulphate were acquired from Hi – media Laboratories, Mumbai, India. All other chemicals were of analytical grade.
Animals
Albino mice of either sex, 7 – 8 weeks of age and 32 -40g of weight were included. The mice were procured from the central animal house of Sri. Kaliswari college, Sivakasi, India. They were housed in poly propylene cages with 12 hours light – dark cycle. Mice were allocated into control and experimental groups and housed 4 – 5 per cage. They were fed standard pellets. All mice were provided food and water ad libitum. The study was carried out after clearance from institutional animal ethics committee.
Experiment
Albino mice were randomly assigned to three groups: Group 1 was control and was injected with vehicle (distilled water) intraperitoneally. Group 2 and 3 animals constituted test groups and were injected intraperitoneally with ceftriaxone (100mg/kg BW and 200mg/kg BW) respectively. All mice were given food and water ad libitum. They were sacrificed next day by cervical dislocation and plasma, erythrocytes and tissue hemolysates were utilised for estimation of lipid peroxidation and various antioxidant activities.
Estimation of Lipid Peroxidation
Lipid peroxidation was assessed by the generation of TBARS.
Estimation of plasma TBARS
Lipid peroxides in plasma were estimated by procedure of Yagi.15 Sample was deproteinised by adding phosphotungstic acid and centrifuged at 3000 rpm for 10 minutes. Thiobarbituric acid was added to precipitate at 90°C for one hour. The resultant pink colour was the index of TBARS. Plasma TBARS was stated as nmol/ml.
Estimation of Erythrocyte TBARS
Erythrocyte TBARS was assessed by the procedure of Donna.16 The erythrocytes were treated with 10% trichloroacetic acid(TCA). The mixture was filtered and thiobarbituric acid was added to a portion of filtrate in the ratio of 1.2:1. The mixture was heated and then cooled. The chromogen so formed was estimated at 535nm. TBARS levels were stated as pmoles/mg Hb.
Estimation of Tissue TBARS
Tissue TBARS was assayed by procedure of Ohkawa et al.17 8.1% sodium dodecyl sulfate and 20% acetic acid were added to sample. It was mixed with TBA. Reaction mixture was heated at 950C for sixty minutes. After addition of distilled water and n-butanol pyridine, it was centrifuged at 4000 rpm for ten minutes. Absorbance was estimated at 535 nm. Tissue TBARS was stated as nmol/mg protein.
GSH Estimation
It was measured according to procedure of Beutler and Kelley.18 The plasma was mixed with TCA and distilled water and centrifuged. 0.3M disodium hydrogen phosphate and DTNB reagent were mixed with the supernatant. Chromogen was estimated at 412 nm. GSH activity was stated as mg/dl of plasma.
Estimation of Glutathione Peroxidase (GPx)
GPx activity was assessed by procedure of Rotruck et al.19 Hemolysate was permitted to react with H2O2 along with GSH, then the remainder of GSH was permitted to react with DTNB. Yellow colour yielded was measured at 412nm. GPx activity was stated as U/g of erythrocyte lysate.
Estimation of SOD
Activity of superoxide dismutase was assessed by procedure of Kakkar et al.20 Diluted solution of hemolysate was mixed with ethanol and chloroform and centrifuged. SOD activity was determined as follows. To 0.5ml of enzyme preparation, sodium pyrophosphate buffer, phenazine methosulphate and NBT were added. NADH was added to start the reaction. Following incubation at 30°C for ninety seconds, glacial acetic acid was mixed to stop the reaction. After addition of n-butanol, centrifugation was done. Colour intensity of the choromogen was measured at 560nm. SOD activity was stated as U/mg of hemolysate.
Estimation of Catalase
Activity of catalase was assessed colorimetrically as per the procedure of Sinha.21 Erythrocyte lysate and H2O2 were added to phosphate buffer. After 90 seconds, dichromate-acetic acid mixture was added. A control with all the reagents other than hemolysate was prepared. The sample was processed in boiling water bath for ten minutes. Colour formed was estimated at 620nm. The activity was stated as U/mg of hemolysate.
Statistical Analysis
Data was represented as mean ± SD. The levels of TBARS in plasma, erythrocytes and tissue and antioxidant enzyme activities were assessed in the control and test groups. The data from ceftriaxone group was analyzed with control group. The results were analyzed using ANOVA and Dunnett’s test as post hoc. p < 0.05 was considered as significant. Data was analyzed by GraphPad prism version 6.05.
Results
Plasma, erythrocyte and tissue TBARS levels and activities of antioxidant enzymes were estimated in the control and experimental groups. TBARS formation was the index of lipid peroxidation. Plasma TBARS level were expressed as nmol/ml. Erythrocyte TBARS values were expressed as pmoles/mg Hb. The tissue TBARS levels were estimated as nmol/mg protein. The values of GSH were expressed as mg/dl. The GPx activity was stated as U/g. The activity of SOD was expressed as U/ mg. The CAT activity was stated as U/mg.
The effect of ceftriaxone on plasma, erythrocyte and tissue TBARS levels in mice are presented in Table 1. Ceftriaxone was considered as an inducer of lipid peroxidation if it increased the levels of plasma, erythrocyte as well as tissue TBARS significantly in comparison with control. In current study, ceftriaxone (100 mg/kg body weight) did not alter TBARS levels in plasma, erythrocytes and tissues in comparison with control. (Table 1, Figure 1) Ceftriaxone (200 mg/kg body weight) has significantly (p< 0.05) increased plasma, erythrocyte and tissue TBARS levels in comparison with control group. (Table 1, Figure 1).
Table 1: Effect of ceftriaxone on plasma, erythrocytes and tissue TBARS levels in mice.
| Treatment group | Plasma TBARS (mean±SD) | Erythrocyte TBARS (mean±SD) | Tissue TBARS (mean±SD) |
| Group 1: Distilled water | 48 ± 4.1 | 37 ± 5.4 | 38 ± 8.1 |
| Group 2: Ceftriaxone (100 mg/kg) | 37 ± 4.5 | 34 ± 3.2 | 44 ± 4.5 |
| Group 3: Ceftriaxone (200 mg/kg) | 79 ± 5.2*** | 71 ± 4.8*** | 89 ± 3.2*** |
Data represented as mean ± SD. n = 6; *p < 0.05, **p<0.01, ***p<0.001 (in comparison with control).
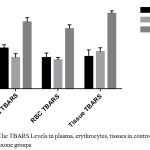 |
Figure 1: The TBARS Levels in plasma, erythrocytes, tissues in control and ceftriaxone groups.
|
The effect of ceftriaxone on antioxidant enzymes activities in mice is presented in Table 2. The activities of antioxidant enzymes – GSH, GPx, CAT and SOD were decreased significantly (p < 0.05) in ceftriaxone (100 mg/kg body weight and 200 mg/kg body weight) treated animals as compared to control animals.(Table 2, Figure 2) There was a dose dependent decrease in the antioxidant enzyme activities.
Table 2: Effect of ceftriaxone on antioxidant enzyme levels in mice.
| Treatment group | GSH | GPX | CAT | SOD |
| Group 1: Distilled water | 77 ± 2.6 | 79±6.24 | 88±6.6 | 86±4.2 |
| Group 2: Ceftriaxone (100 mg/kg) | 54±6.1** | 41±6.24** | 49±5.8** | 59±6.5** |
| Group 3: Ceftriaxone (200 mg/kg) | 48±2.7*** | 37±6.0*** | 47±5.7** | 51±4.2*** |
Data represented as mean ± SD. n = 6; *p < 0.05, **p<0.01, ***p<0.001 (in comparison with control).
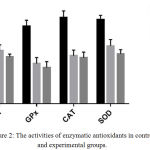 |
Figure 2: The activities of enzymatic antioxidants in control and experimental groups.
|
Discussion
Lipid peroxidation byproducts can result in marked damage to structure and functional integrity of cell membranes.22 Antioxidants (GSH, SOD, CAT, GPx) scavenge the byproducts of lipid peroxidation.23 But an imbalance between the antioxidants and lipid peroxidation byproducts can lead to cell and tissue damage.24,25
Drug induced lipid peroxidation may be related to the toxic potential of the drugs. The capability of various antioxidants in reducing this phenomenon, may be useful in preventing drug-induced toxicity.26
In current study, the influence of ceftriaxone on lipid peroxidation as well as antioxidants levels in mice was evaluated at 100 mg/kg body weight and 200 mg/kg body weight. TBARS generation was the index of lipid peroxidation. The level of TBARS was estimated in plasma, erythrocytes and tissues of mice in the control and experimental groups. The antioxidant enzyme activities (GSH, SOD, CAT, GPx) were also estimated.
In current study, ceftriaxone, 100 mg/kg body weight did not alter the plasma, erythrocyte and tissue TBARS levels in comparison with control.(Table 1, Figure 1) Ceftriaxone, 200 mg/kg, has significantly (p< 0.05) increased the levels of TBARS in plasma, erythrocytes and tissues in comparison with control group.(Table 1, Figure 1) Hence ceftriaxone in higher dose (200 mg/kg body weight) induces lipid peroxidation in albino mice. Activities of antioxidant enzymes – GSH, GPx, CAT and SOD were decreased significantly (p < 0.05) by ceftriaxone (100 mg/kg body weight and 200 mg/kg body weight) compared to control.(Table 2, Figure 2) There was a dose dependent decrease in the antioxidant enzyme activities.
These results are in conformation with the study done by Chakaraborty S et al, who reported that ceftriaxone significantly induced lipid peroxidation in goat liver homogenate and ascorbic acid could significantly arrest this process.27 Roy K et al also reported that ceftriaxone induced lipid peroxidation in goat whole blood and it was inhibited by glutathione and probucol.28
However, several studies have reported the contrary. Dwivedi VK et al reported that ceftriaxone and sulbactam along with VRP 1034 potently scavenges free radicals and chelates metal ions in cadmium toxicity.29 In the study by Amin B et al, ceftriaxone reduced oxidative stress and apoptosis in rat model of neuropathic pain.3 According to study of Akina S et al ceftriaxone and selegiline possess neuroprotective effect in scopolamine induced cognitive impairment by antioxidant mechanisms.31
Conclusion
According to the results of our study, ceftriaxone has the potential for lipid peroxidation induction and to reduce the activities of the enzymatic antioxidants in mice. However, further studies are necessary to establish whether ceftriaxone induced lipid peroxidation may be responsible for its toxic effects. It is prudent to exercise caution during the clinical use of ceftriaxone.
Acknowledgements
We thank Dr. Kolanjiappan Kaliyaperumal for his guidance and support.
Conflict of Interest
There is no conflict of interest.
Ethical Approval
The study is approved by the Institutional Animal Ethics committee.
References
- Halliwell B., Gutteridge J. M. C. Free radicals in biology and medicine. 5th edition, Oxford university press, Oxford. 2015:10-15.
CrossRef - Roy K., Saha A., De K., Sengupta C. Evaluation of probucol as suppressor of Ceftizoxime induced lipid peroxidation. Acta Poloniae Pharmaceutica – Drug research. 2002;59(3):231-34.
- Halliwell B., Chirico S. Lipid peroxidation its mechanism, measurement and significance. Am Clin Nutr. 1993;57(5):715-24.
CrossRef - Yu B. P. Cellular defences against damage from reactive oxygen species. Biol Rev. 1994;74:139-162.
- Ray G and Husain S. H. Oxidants, antioxidants and carcinogenesis. Indian J. Exp. Biol. 2002; 40:1213-32.
- Vijayakumar D., Suresh K and S. Manoharan. Lipid peroxidation and antioxidant status in blood of rheumatoid arthritis patients. Indian Journal of Clinical Biochemistry. 2006;21(1):104-108.
CrossRef - Gutteridge J. M. Lipid peroxidation and antioxidants as bio markers of tissue damage. Clin Chem. 1995;41:1819-1828.
- Toyokuni S. Reactive oxygen species‐induced molecular damage and its application in pathology. Pathol. Int. 1999;49(2):91-102.
CrossRef - Dorr R. T. Cytoprotective agents for anthracyclines. Semin. Oncol. 1996;23(4):23-34.
- Naito Y., Yoshikawa T., Yoshida N., Kondo M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Drug Dis. Sci. 1998;43(9):30-34.
- Ray S., Roy K And Sengupta C. Evaluation of protective effects of water extract of spirulina platensis (blue green algae) on cisplatin-Induced Lipid Peroxidation. Indian J. Pharm. Sci. 2007;69(3):378-83.
CrossRef - Ulubas B., Cimen M. Y., Apa D. D., Saritus E., Muslu N., Cimen O. B. The protective effect of acetyl salicylic acid on free radical production in cisplatin induced nephrotoxicity an experimental rat model. Drug Chem Toxicol. 2003;26:259-70.
CrossRef - Ali B. H., Gentamicin nephrotoxicity in humans and animals some recent research. General Pharmacology. 1995;26 (7):1477–1487.
CrossRef - Sivachandran M and Hariharan P. Gentamicin Induced Oxidative Stress on Renal Antioxidant Parameters and its Ameiloration by Andrographis Paniculata in Rats. Inter J Agri Biosci. 2012;1(1):11-15.
- Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337-51.
CrossRef - Donna SK. The thiobarbituric acid test applied to tissues from rats treated in various ways. J. Biol Che. 1950;182:415–419.
- Ohkawa H., Ohishi N., Yagi K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Analytical biochemistry. 1979;95:35l-358.
CrossRef - Baitler E., Ketly B. M. The effect of sodium nitrate on RBC glutathione experimental. 1963:19:96-97.
- Rotruck J. T., Pope A. L., Gantha H. E., Swanson A. B., Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1984;179:558-590.
- Kakkar P., Das B., Viswanthan P. N. A modified spectrophotometric assay of superoxide dismutase. Ind J. Bio Chem Bio Phys. 1984;21:130-132.
- Sinha K. A. Colorimetric assay of catalase. Bio. Chem. 1972;47:389-394.
- Kolanjiappan K., Manoharan S., Kayalvizhi M. Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin Chim Acta. 2002;326:143-9.
CrossRef - Manoharan S., Kolanjiappan K., Suresh K & Panjamurthy K. Lipid peroxidation & antioxidants status in patients with oral squamous cell carcinoma. Indian J Med Res.December. 2005;122:529-34.
- Nagini S., Saroja M. Circulating lipid peroxides and antioxidants as biomarkers of tumour burden in patients with oral squamous cell carcinoma. J Biochem Mol Biol Biophys. 2001;5:55-9.
- Guyton K. Z., Kensler T. W. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523-44.
CrossRef - Ray S., Roy K And Sengupta C. Cisplatin-Induced Lipid Peroxidation and Inhibition with Ascorbic Acid. Indian J. Pharm. Sci. 2006;68(2):199-204.
CrossRef - Chakaraborty S., Bhuti P. D., Ray S., Sengupta C., Roy K. A study on ceftriaxone – induced lipid peroxidation using 4-hydroxy-2-nonenal as model marker. Acta Pol Pharm. 2005;62(2):141-3.
- Roy K., Saha A., De K., Sengupta C. Ceftriaxone induced lipid peroxidation and its inhibition with various antioxidants: Part II. Evaluation of glutathione and probucol as antioxidants. Acta Pol Pharm – drug research. 2000;57(5):385-90.
- Dwivedi V. K., Bhatanagar A., Chaudhary M. Protective role of ceftriaxone plus sulbactam with VRP1034 on oxidative stress, hematological and enzymatic parameters in cadmium toxicity induced rat model. Interdiscip Toxicol. 2012;5(4):192–200.
CrossRef - Amin B., Hajhashemi V., Abnous K., Hosseinzadeh H. Ceftriaxone, a beta-lactam antibiotic, modulates apoptosis pathways and oxidative stress in a rat model of neuropathic pain. BioMed Research International. 2014;1-9.
CrossRef - Akina S., Thati M., Puchchakayala G. Neuroprotective Effect of Ceftriaxone and Selegiline on Scopolamine Induced Cognitive Impairment in Mice. Advances in Biological Research. 2013;7(6):266-275.








