Ritesh Jain1*, K. Nandakumar1, Vishal Srivastava1, Santosh Kumar Vaidya1, Shailesh Patel1, Pradeep Kumar² and Lalit Kumar
¹Department of Pharmacology, P.E.S College of Pharmacy, Bangalore.
²Department of Pharmaceutical Analysis, P.E.S. Collage of Pharmacy, Bangalore, (India).
³Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, Karnataka.
Abstract
Anticonvulsant activity of the aqueous extracts of Aquilaria agallocha Roxb. family Thymelaeceae was studied in Swiss albino mice using the Pentylene tetrazole (PTZ) and Maximal electroshock (MES) convulsion.In Pentylene tetrazole (PTZ) induced convulsion model, aqueous extracts of Aquilaria agallocha (AQAA) 300 and 600 mg/kg, p.o doses significantly increased both the onset of clonus and tonic convulsions induced by PTZ. The effect was dose dependent. In Maximal electroshock (MES) induced convulsion model, 300 mg/kg dose of AQAA exhibited significant anti convulsant effect, there is significant decrease in the duration of extensor phase with increase in the latency of clonus convulsion. The 600 mg/kg of AQAA also increased the latency of clonus in both acute and chronic studies and decreased the duration of extensor phase. From the experimental study it can be concluded that aqueous extract of heartwood of Aquilaria agallocha had exhibited significant anticonvulsant activity in mice.
Keywords
Aquilaria agallocha; Convulsion; Pentylene tetrazole (PTZ); Maximal electroshock (MES)
Download this article as:| Copy the following to cite this article: Jain R , Nandakumar K , Srivastava V , Vaidya S . K , Patel S , Kumar P , Kumar L . Anticonvulsant Activity of Aqueous Extract of Aquilaria Agallocha .Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Jain R , Nandakumar K , Srivastava V , Vaidya S . K , Patel S , Kumar P , Kumar L . Anticonvulsant Activity of Aqueous Extract of Aquilaria Agallocha .Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=251 |
Introduction
Seizures are discrete, time-limited alterations in brain function including changes in motor activity, autonomic function, consciousness, or sensation, that result from an abnormal and excessive electrical discharge of a group of neurons within the brain. It has been shown to affect several brain activities and promote long-term changes in multiple neural systems.1
Epilepsy is the most common chronic disorder of the central nervous system (CNS) manifested by recurrent unprovoked seizures that affect approximately 1% of the U.S. population.2 It is estimated that there are 50 million people with epilepsy world wide and the majority of cases are in developing countries. This disorder, if untreated, can lead to impaired intellectual function or death and is typically accompanied by psychopathological consequences such as loss of self-esteem.3
Agar wood (Aquilaria agallocha) is one of such plant that has been used in Ayurvedic formulation for the treatment of mental illness.4 Traditionally it is known to strengthen the brain, Aquilaria agallocha has to enhance cerebral function, balance the mind or body connection and the nervous system. In Japanese system of medicine it has been used as a sedative and anxiolytic agents. It is reported to possess CNS depressant activity in mice.5
The plant was traditionally used as laxative, diuretic, stomachic, used in chronic diarrhoea, disease of the liver and intestines, bronchitis, asthma, vomiting, gout, rheumatism and headache. In China the wood is used as tonic, stimulant, carminative, aphrodisiac, in the treatment of snakebite and scorpion-sting. (charaka, sushruta).6
However there is lack of scientific data regarding its anticonvulsant activity. Hence it was thought worthwhile to study the aqueous extract of Aquilaria agallocha for anticonvulsant activity in mice.
Material and Methods
Plant Material
Heart wood of Aquilaria agallocha was purchased from Green Pharmacy, Pune and was authenticated by Green Pharmacy, Pune, India Vide letter no.GPP/411/2004.
Drugs
Diazepam (Ranbaxy laboratories limited, HMTD Textiles, Mumbai, India)
Phenytoin (Sun Pharmaceutical India.ltd, Halol, Gujarat, India)
Pentylene tetrazole (St.Louis, MO USA) were used for the pharmacological investigation.
Other chemicals used for extraction and phytochemical investigation were of analytical grade from S.D fine chemicals, Mumbai, India.
Animals
Swiss albino mice (18-22) purchased from Bioneeds, Tumkur were maintained in the animal house of PES College of Pharmacy, Bangalore for experimental purpose. Then all the animals were acclimatized for seven days under standard husbandry conditions, i.e.; room temperature of 25 ± 10 C; relative humidity 45-55% and a 12:12h light/ dark cycle. The animals had free access to standard rat pellet (Pranav Agro Industries Ltd, Mysore feeds, India), with water supplied ad libitum under strict hygienic conditions. The approval of the Institutional Animal Ethical Committee (IAEC) of P.E.S College of Pharmacy Bangalore (Karnataka) was taken prior to the experiments. All the protocols and the experiments were conducted in strict compliance according to ethical principles and guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Preparation of aqueous extract
The plant material was dried, powdered and it was extracted with petroleum ether, 95 % ethanol and water successively. Aqueous extraction was done by maceration process for 72 hours with chloroform water. The solvents were removed by vacuum distillation and extracts were dried below 500C. The percentage yield of the aqueous extract was 2% w/w.
Preliminary phytochemical investigations
The preliminary phytochemical investigations were carried out with aqueous extract of Aquilaria agallocha for qualitative identification of phytochemical constituents present with the extract. Tests were carried out by following standard methods.
Acute oral toxicity studies
Female Swiss albino mice (18-22 g) were individually identified and allowed to acclimate to the laboratory conditions for 7 days before the start of the study. Only one mouse received a dose at a particular time. First animal received a dose of 55 mg/kg, p.o. Animal was observed for 48 hours after injection, for any toxicity signs, survival or death. If the first animal died or appeared moribund, the second animal received a lower dose (17.5mg/kg). The dose progression or reduction factor was 3.2 times of the previous dose. If no mortality was observed in the first animal then the second animal received a higher dose (175 mg/kg). Dosing of the next animal was continued depending on the outcome of the previously dosed animal for a fixed time interval (48 hour). The test was stopped when one of the stopping criteria was met:
5 reversals occur in any 6 consecutive animals tested.
3 consecutive animals died at one dose level.
Survived animals were observed for long-term outcomes for a period of 14 days. The LD50 values were calculated using AOT 425 software (Environmental Protection Agency, USA) based on the short term (48 hour) and long term out come (14 days).7
Determination of Anticonvulsant Activity
Pentylenetetrazole induced convulsion (PTZ) induced convulsions8
Male albino mice weighing between 20-25g were divided into five groups each comprising of six animals and were treated as follows:
Group I : Control (3% Tween-80 p.o.)
Group II : Standard drug (diazepam, 5 mg/kg p.o.)
Group III : Low dose of AQAA (300 mg/kg p.o.)
Group IV : Higher dose of AQAA (600 mg/kg p.o.)
(AQAA: Aqueous extract of Aquilaria agallocha.)
Experimental Procedure
One hour after vehicle/standard/extract treatment PTZ (pentylenetetrazole 80 mg/kg) was administered i.p. to all groups of mice. Each animal was placed in to individual plastic cage and were observed initially for 30min and later up to 24 hrs. The following parameters were recorded during test session of initial 30min and up to 24 hrs: Latency (onset of clonus),Onset of tonic convulsions, Status of animal after 30 minutes, Status of animal after 24 hrs, Percent protection.
Maximal Electroshock (MES) induced convulsions9
Male albino mice weighing between 20-30g were divided into five groups, each comprising of six animals and were treated as follows:
Group I : Control (3% Tween-80 p.o.)
Group II : Standard drug (Phenytoin 25 mg/kg p.o.)
Group III : Low dose of AQAA (300 mg/kg p.o.)
Group IV : Higher dose of AQAA (600 mg/kg p.o.)
Experimental Procedure
A 60mA current was delivered transauricularly for 0.2 sec in mice via small alligator clips attached to cornea. For recording various parameters, mice were placed in a rectangular plastic cage with an open top, permitting full view of animal’s motor responses to seizure in the pilot study of various phases of convulsions.
The following parameters were recorded during 30 min.test session:Tonic flexion,Tonic extension,Clonus convulsions,Stupor and Percent protection
Sataical Analysis
The values were expressed as mean ± SEM from 6 animals. The results were subjected to statistical analysis by using ANOVA followed by Tukey-Kramer test to calculate the significance difference if any among the groups. P<0.05 was considered as significant.
Results
Preliminary phytochemical screening
The aqueous extract of Aquilaria agallocha (AQAA) subjected for preliminary phytochemical investigation and found to contain flavonoids, tannins, saponins, carbohydrates and tri terpenoids.Acute Oral Toxicity Study: Different doses of AQAA were screened for their oral toxicity and it was found that AQAA was safe up to the dose of 5 g/kg.
Assessment of anticonvulsant activity
PTZ (Pentylene tetrazole) induced convulsions
AQAA was subjected for anti convulsant effect using PTZ induced convulsions model in mice.
The study revealed that lower dose (300mg/kg) of AQAA had significantly altered onset of clonus and onset of tonic seizures, but higher dose exhibited much significant anticonvulsant effect by increasing latency, onset of tonic seizures and 33.33% of animals were survived with lower dose and 83.33% survived with higher dose at 30min interval but only 16.66% of animals survived after 24hrs with lower dose. (Table 1, figure 1 & 2.)
Table 1: Effect of AQAA on Pentylenetetrazole induced convulsions
|
S.NO |
TREATEMENT |
LATENCY (Onset of clonus) (Sec) |
ONSET OF TONIC (Sec) |
STATUS OF ANIMAL (24 hrs, No. of animals Alive)
|
% PROTECTION (24h) |
|
1 |
Control (3%tween80) |
49.33 ± 5.32 |
368.33 ± 17.97 |
0 |
0 |
|
2 |
Diazepam (5 mg/kg) |
No clonus |
No Tonic |
ALL |
100 |
|
3 |
AQAA (300 mg/kg, po)
|
175 ± 27.29** |
672 ± 69.46 ** |
1 |
16.66 |
|
4 |
AQAA (600 mg/kg, po)
|
265 ± 27.29*** |
980 ± 131.15 *** |
5 |
83.33 |
(Observation period: 5min for all parameters) Values are expressed as mean ± SEM, from 6 mice.
Significant at **P<0.01 and ***P<0.001 as compare to control using one-way ANOVA followed by
Turkey’s post hoc test.
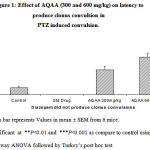 |
Figure 1: Effect of AQAA (300 and 600 mg/kg) on latency to produce clonus convulsion in PTZ induced convulsion. |
 |
Figure 2: of AQAA (300 and 600 mg/kg) on latency to produce tonic convulsion in PTZ induced convulsion |
MES (Maximal Electro Shock) induced convulsions
AQAA was subjected for its anticonvulsant effect using MES induced convulsion model. In this study, two dose of AQAA (300 and 600mg/kg) when administered as single doses, lower dose had exhibited a significant anti convulsant effect by altering the duration of tonic extensor but no significant difference in latency of clonus with 33.33% of protection against convulsions. Higher dose also exhibited significant effect on duration of tonic extensor phase and also exhibited significant effect with latency of clonus with 83.33% of protection from convulsions (Table 2, Figure 3 & 4.)
Table 2: Effect of AQAA on Maximal Electroshock induced convulsions
|
S.NO |
TREATEMENT |
DURATION OF TONIC FLEXION (Sec) |
DURATION OF TONIC EXTENSOR (Sec) |
% PROTECTION
(24h) |
|
1 |
Control (3% tween 80) |
NO |
14.17 ± 0.872 |
16.66 |
|
2 |
Phenytoin (25 mg/kg) |
5.83 ± 0.9804
|
NO*** |
100 |
|
3 |
AQAA (300 mg/kg, po) |
NO |
8 ± 0.577
|
33.33 |
|
4 |
AQAA (600 mg/kg, po) |
NO
|
7.83 ± 0.60* |
83.33 |
(Observation period: 5min for all parameters)
Values are expressed as mean ± SEM, from 6 mice.
Significant at *P<0.05 and ***P<0.001 as compare to control using one-way
ANOVA followed by Turkey’s post hoc test.
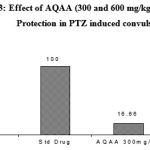 |
Figure 3: Effect of AQAA (300 and 600 mg/kg) on percentage Protection in PTZ induced convulsion. |
 |
Figure 4: Effect of AQAA (300 and 600 mg/kg) on duration of extensor phase in MES induced convulsion |
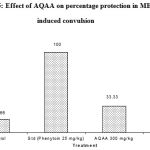 |
Figure 5: Effect of AQAA on percentage protection in MES induced convulsion |
Discussion
Different doses of AQAA were screened for there oral toxicity and it was found that AQAA was not toxic up to the dose of 5g/kg based on the toxicity studies. Two doses 300 and 600 mg/kg dose were selected to determine anticonvulsant activity of AQAA.
In this study, with PTZ induced convulsion model, parameters like latency, onset of tonic convulsions and percent protection were observed after treatment with AQAA. Diazepam, 5 mg/kg had prevented tonic and clonus convulsions and offered 100% protection. AQAA 300 mg/kg dose has significantly increased the latency of clonus and tonic phase and offered 16.66 percentage protection. But higher 600 mg/kg significantly increased the onset of both clonus and tonic phases and shown 83.33 percentage protection.
Pentylenetetrazole is a selective blocker of the chloride ionophore complex to the GABA-A receptor, and after repeated or single dose administration leads to a decrease in GABAergic function and to the stimulation and modification of density or sensitivity of different glutamate receptor subtypes in many brain regions. Pentylenetetrazole may also trigger a variety of biochemical processes including the activation of membrane phospholipases, proteases, and nucleases. Marked alteration in membrane phospholipid metabolism result in the liberation of free fatty acids (FFAs), diacylglycerols, eicosanoids, lipid peroxides and free radicals.10 These results suggest that AQAA with 300 and 600mg/kg doses has exhibited significant anti convulsant effect by increasing both the onset of clonus and onset of tonic convulsions and greater effect was recorded with higher dose but not with lower dose. Hence these drug may able to modulate the function of GABA (or) glutamate receptor.
In this study of MES-induced convulsion model, lower dose (300mg/kg) as well as higher dose (600mg/kg) of AQAA both has offered anti convulsant effect against electroshocks induced convulsions. In this study, phenytoin 25mg/kg had prevented clonus convulsions with a 100% protection and produced tonic flexion convulsion. AQAA with dose of 300 mg/kg had shown a significant anti convulsant effects by altering the duration of extensor phase but not onset of clonus and offered 33.33%, protection without producing flexion convulsions. But the dose of 600 mg/kg has significantly altered both duration of extensor phase as well as onset of clonus and offered 83.33%, protection without producing flexion convulsions. In this study it was observed that if, the tonic flexion convulsions produced, onset of clonus and percent protection will increase with decrease the duration of tonic extensor.
These results suggest that the lower dose (300 mg/kg) of AQAA has less anti convulsant effect than higher dose (600mg/kg) of AQAA; which may be due to the more sedative effect with higher (600mg/kg) dose.
Since AQAA has able to protect PTZ and MES induced convulsions, the drug may possess anti-convulsant activity of generalized in nature.
From the experimental study it can be concluded that aqueous extract of heartwood of Aquilaria agallocha had exhibited significant anticonvulsant activity in mice.
Phytoconstituents like flavonoids and saponins were reported for their anxiolytic and anti convulsant effect and these two were present in aqueous extract. These active principles can be accounted for both anxiolytic and anti convulsant effect.
However, further studies including toxicological evaluation and the use of more purified fractions are necessary to confirm and extend the present findings.
References
- Hauser W.A., Engel J., Pedley T.A., Epilepsy: A comprehensive Textbook, Lippincott-Raven, 47 (1997).
- Sibat H.F., Romera A.I. and Valdes LDEF, The Inter J Neurol., 2005;4:1.
- Shindikar A.V, Khan F, Viswanathan C.L. Design, synthesis and in vivo anticonvulsant screening in mice of novel phenylacetamides. Europ J Med Chem 2006; 41:786-92.
- Okugava H, Ueda R, Matsumoto K, Karvanishi K, Kato A. Effects of agar wood extracts on the CNS in mice. Planta Med 1993;59:32-6.
- He ML, Qi SY, Hu L. Rapid invitro propagation of medicinally important Aquilaria. J Zhejiang Univ 2005;6(8):849-52.
- Kirtikar KR., Basu BD. Indian medical plants Blaster E., Caius JF., Bhaskar KS. (Eds). Periodical Experts Book Agency, New Delhi.1991;3:2171-72.
- OECD 2001-gudeline on acute oral toxicity (AOT) Environmental health and safety monograph series on testing and adjustment No.425.
- Khosla P., Pandhi P. Anticonvulsant effect of nimodipine alone and in combination with diazepam on PTZ induced convulsions. Ind J Pharmacol 2001;33:208-211.
- Swinyard EA., Brown WC., Goodman LS. Comparitive assays of antiepileptic drugs in mice and rats. J Pharmacol Exp Therap 1952;106:319-30.
- Ilhan A, Iraz M, Kamisli S, Yigitoglu R. Pentylenetetrazol-induced kindling seizure attenuated by Ginkgo biloba extract in mice. Progress in Neuro-Psychopharmaco & Biology Psychiatry; 2006.







