Manuscript accepted on :06-Dec-2018
Published online on: --
Plagiarism Check: Yes
Reviewed by: Akmal El-Mazny
Second Review by: Nicolas Padilla
Centre for Craniofacial Diagnostics and Biosciences, Faculty of Dentistry, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Corresponding Author E-Mail: leongxinfang@ukm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/1555
Abstract
Untreated hypertension is a major cause for a wide array of diseases affecting cardiovascular system. Oxidative stress has been implicated in the development of hypertension. The impairment between the balance of antioxidants and pro-oxidants contributes to the elevation of blood pressure. Over generation of free radicals produces a decreased bioavailability of nitric oxide. Eventually, this will cause a rise in total peripheral resistance and lead to endothelial dysfunction. Noticeable symptoms are usually experienced when hypertension enters the advanced stage with lifelong health complications. Hypertensive patients are required to take medications for indefinite period of time to prevent further deterioration. Many of these therapeutic agents are costly and associated with unwanted side effects. Curcuma longa (CL) or turmeric is one of the alternative herbs which confers medicinal properties. This review aims to summarise the effects of CL and its active constituents on blood pressure derived from preclinical and clinical published articles. Studies documented that CL and its active constituents could reduce blood pressure. These were achieved by antioxidant, anti-inflammatory activity, calcium (II) ion concentration interference, β2-adrenergic receptor activation, and renin-angiotensin system inhibition. There is a prospect for CL in the management of hypertension. However, limited researches of CL have been conducted on human. Thus, more well-planned studies should be carried out to ascertain its effectiveness.
Keywords
Angiotensin; Antioxidant; Curcuma Longa; Hypertension; Inflammation
Download this article as:| Copy the following to cite this article: Leong X. F. The Spice for Hypertension: Protective Role of Curcuma Longa. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Leong X. F. The Spice for Hypertension: Protective Role of Curcuma Longa. Biomed Pharmacol J 2018;11(4). Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24808 |
Introduction
High blood pressure (BP) or hypertension is considered as the prevalent risk factor for the pathogenesis of cardiovascular diseases (CVD) such as myocardial infarction, stroke, and cardiac failure.1,2 The main obstacle for healthcare system in many countries is the high incidence and prevalence of hypertension, which affects chiefly the older adults; with increased risk of all-cause mortality.3,4
According to the Eight Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 8), a person who is diagnosed of having hypertension has been redefine to have systolic blood pressure (SBP) between 130 and 139 mm Hg or diastolic blood pressure (DBP) ranging 80 to 89 mm Hg.5 This new threshold would lead to more people to be categorised as having hypertension compared to the previous BP reading in JNC 7, 140 to 159 mm Hg and 90 to 99 mm Hg for SBP and DBP, respectively.6 Under JNC 8 classification for hypertension, those with clinical CVD are required to take medications and accompanied by lifestyle changes.5
Therefore, it is better to prevent than to cure with long-term antihypertensive medications. Prolonged intake of medications is often associated with undesired side effects. Hence, this has prompted the researchers to explore alternative treatment with comparable efficacy, affordable cost, and minimal adverse effects. Interest and demand for using plant-based traditional medicines in treating and preventing CVD have been growing. The reason may be attributed to easy availability, efficacy and cultural acceptability. Curcuma longa (CL), or commonly known as turmeric, originates from southeast India and is extensively cultivated in tropical areas of South Asia. CL belongs to the ginger family, also known as Zingiberaceae. It is an aromatic perennial herbal plant. Its tuberous rhizome has been widely used in medicinal, culinary and dyeing purposes.7
Turmeric has been used as a food colour additive and a dyeing agent in textile industry due to its distinct yellow colour. It is mainly consumed in curry dishes. In addition, it is available in different forms for various uses such as drinks, capsules, tablets, powder, ointments, and soaps.8 Curcumin extracted from the rhizome has been documented to exhibit potent pharmacological properties such as antioxidant,9,10 anti-inflammatory,11,12 antimicrobial,13,14 anticarcinogenic,15,16 hypoglycaemic,17 and hepatoprotective effects.18 Thus, the aim of this review paper was to compile the available evidence on the cardiovascular protective actions of CL against high BP besides of the potential pathways involved.
Literature Search
Literature search was performed with two online databases, namely PubMed and Scopus using keywords “curcumin” OR “Curcuma longa” OR “curcuminoids” AND “hypertension”. The electronic search was carried out from 1st July 2018 to 31st July 2018. Relevant original research articles written in English were retrieved. Preclinical and clinical studies were included in this review.
Curcuma longa (CL)
Among the Curcuma species, CL has the highest amount of curcuminods.19 The three main curcuminoids found in the CL are curcumin, demethoxycurcumin and bisdemethoxycurcumin.20,21 Among these curcuminoids, curcumin has been studied extensively. Curcumin is poorly absorbed when given orally. Although curcumin has low oral bioavailability, it possesses pronounced biological activities. It is rapidly metabolised to form major metabolites such as tetrahydrocurcumin and hexahydrocurcumin which were identified in plasma and urine.22-24
The effects of CL on blood pressure in animal studies
Goto et al. evaluated the effect of yellow turmeric, CL and white turmeric, Curcuma zedoaria (CZ) on vasomotion and haemorheology in adult male spontaneously hypertensive rats (SHR).25 The animals were fed with normal rat chow or chow fortified with 3% weight/weight (w/w) of CL, 1% w/w of CZ, 3% w/w of CZ or 100 mg/kg/day of captopril in drinking water, respectively, for 12 weeks of study duration. It was reported that 3% w/w of CZ was more effective as a hypotensive agent than CL. This is because the latter merely showed a trend in reducing SBP while the former with a significant reduction of SBP compared to the control group. In addition, ingestion of 3% w/w of CZ showed a greater increment in endothelium-dependent relaxation following addition of acetylcholine (ACh). Captopril, a positive control showed a similar pattern of activities. On the other hand, rats fed with 3% w/w of CZ diet exhibited a marked reduction in aortic contraction in response to xanthine oxidase. This enzyme is responsible for the production of reactive oxygen species (ROS). Both 3% w/w of CL and CZ groups significantly reduced the low shear stress of whole blood viscosity. However, this protective effect was not observed in the rats given with captopril.
Hypotensive and vasorelaxant activities of the methanolic extract of CL (MECL) were studied in male Wistar normotensive rats.26 The mean arterial pressure was significantly reduced at the given dosage of 20 mg/kg and 30 mg/kg. Meanwhile, the heart rate was significantly lessened following the dose from 1 mg/kg to 30 mg/kg of MECL administered intravenously. The increasing concentration of MECL from 1 µg/mL to 1000 µg/mL attenuated the precontraction induced by phenylephrine (PE) and potassium chloride (KCl) in both the intact and denuded isolated superior mesenteric rings. Adaromoye et al. then conducted a series of experiment to inspect the possible mechanism of relaxant action elicited by MECL.26 Endothelium-denuded mesenteric rings were precontracted with PE after being pre-incubated with four different agents, namely glibenclamide, barium chloride, tetraethylammonium and 4-aminopyridine, respectively, to inhibit potassium ion (K+) channels. However, these inhibitors did not affect the vasorelaxation induced by the increasing concentration of MECL in the rings precontracted with PE.
Effect of MECL on contraction induced by calcium chloride (CaCl2) also being examined.26 It was reported that CaCl2 evoked contraction in a concentration-dependent manner. Presence of MECL from 1 µg/mL to 1000 µg/mL significantly suppressed the contraction in response to CaCl2. In order to determine whether the MECL could disturb calcium (II) ion (Ca2+) release intracellularly, the denuded mesenteric rings were precontracted with KCl in a Ca2+-free condition. Additionally, the rings were eventually exposed to PE or caffeine.26 The induced-contractile responses were then evaluated with the cumulative concentration of MECL. The obtained results revealed that MECL was capable to attenuate the transient contraction elicited by PE in endothelium-denuded mesenteric rings. However, the similar findings were not to be observed when tested with caffeine.
Hlavačková et al. performed a study to determine the abilities of curcumin and combination of curcumin and piperine on the BP and aorta remodelling in adult male Wistar rats.27 Hypertension was induced by administration of Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME). The BP was measured weekly and the isolated thoracic aorta was stained for morphological examination. The increased of BP by L-NAME was partially prevented by administration of 100 mg/kg/day of curcumin. Despite of that, combination of curcumin and piperine showed less significant results on BP. Curcumin was reported to reduce the myofibrils and increase the actin as well as the elastin content in the aortic media of the nitric oxide (NO)-deficient rats. Thus, curcumin was more capable than concurrent use of curcumin and piperine to prevent aorta remodelling with morphological changes in the vascular wall induced by hypertension.
Tetrahydrocurcumin (THC) is one of the major metabolites of curcumin. Adult male Sprague-Dawley rats were fed with L-NAME in drinking water for three weeks.28 Curcumin or THC (50 mg/kg/day and 100 mg/kg/day) was fed to the rats simultaneously with L-NAME. Both curcumin and THC decreased BP, vascular resistance and improved vascular responsiveness in hypertensive rats. Moreover, curcumin or THC reduced the plasma levels of malondialdehyde (MDA) and protein carbonyl in hypertensive rats as well as the production of superoxide in the aorta. Besides, both curcumin and THC were able to prevent the depletion of blood glutathione (GSH) and to restore the redox status of the L-NAME-treated rats. Treatment with curcumin or THC also increased endothelium nitric oxide synthase (eNOS) protein expression in aorta and plasma nitrate/nitrite levels. Interestingly, the antihypertensive and antioxidant effects of THC was greater than curcumin.
In another study by Nakmareong et al., the rats were given L-NAME for five weeks instead of three weeks.29 Furthermore, a dose of 50 mg/kg/day or 100 mg/kg/day of THC was intragastrically administered to the rats at the last two weeks once the hypertensive stated had been established with L-NAME. The same parameters were carried out as performed by Nakmareong et al.28 However, Nakmareong et al. tested the aortic elasticity29 instead of aortic reactivity28. The findings obtained in this study29 were in agreement with the earlier study28. In addition, THC reduced thoracic aortic wall thickness and stiffness in the rats receiving L-NAME.29 THC, especially the higher dose showed a greater protective action in hypertensive rats.28,29 Despite of that, both doses of THC or curcumin had no effect on normotensive rats.28,29 These obtained results confirmed the deficiency of NO production, which eventually led to the elevation of BP following administration of L-NAME.
Boonla et al. used a silver clip to clip the left renal artery of male Sprague-Dawley rats to create a 2 kidney-1 clip (2K-1C) model with renovascular hypertension.30 The rats were gavaged with two different doses of curcumin (50 mg/kg/day and 100 mg/kg/day) for six weeks.30 At the end of the treatment, curcumin reduced hindlimb vascular resistances and arterial BP as well as increased hindlimb blood flow in 2K-1C hypertensive rats. There was an impairment of endothelium-dependent vasorelaxation in response to ACh when tested on isolated thoracic aortic rings from hypertensive rats compared to the sham control groups. However, curcumin administration dose-dependently increased the relaxant action. Treatment with or without curcumin either in the sham or hypertensive groups did not affect the endothelium-independent relaxation elicited by sodium nitroprusside. The endothelial dysfunction in the 2K-1C hypertensive rats was restored by curcumin with the increased of plasma nitrate/nitrite levels and e-NOS protein expression in the thoracic aorta. Furthermore, curcumin treatment ameliorated the increased of superoxide production in the carotid artery and the oxidative stress markers such as MDA and protein carbonyl in the plasma of 2K-1C rats.
This renovascular hypertension caused an elevation in the plasma angiotensin-converting enzyme (ACE) level accompanying with an increase of a regulatory subunit of reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, p47phox in thoracic aorta.30 In contrast, administration of curcumin shown a reduction in both the ACE level and the protein expression of p47phox NADPH oxidase subunit. Boonla et al. also revealed that hypertension induced structural changes and remodelling in thoracic aorta of the 2K-1C rats.30 Aortic wall hypertrophy and hyperplasia were observed in parallel with the increased of smooth muscle actin, collagen and elastin contents. In addition, there was an increase of matrix metalloproteinases (MMP) levels including MMP2 and MMP9 in the aorta of the 2K-1C hypertensive rats. Curcumin significantly reversed the abnormalities of vascular structure and remodelling in this study. The protective effect of curcumin follows a dose-dependent manner, especially the higher dose which exhibited a greater beneficial action in 2K-1C hypertension.
Akinyemi et al. studied the effect of CL on ACE and arginase activities in adult male Wistar rats.31 The rats were divided into five groups: normotensive control, hypertensive, hypertensive with atenolol (β1-adrenergic receptor antagonist), normotensive with CL and hypertensive with CL, respectively. The rats were induced by L-NAME for 10 days to become hypertensive. The study duration was 24 days. Ingestion of L-NAME produced a significant rise in SBP compared to the normotensive rats. On the other hand, rats fed with diet supplemented with 4% of CL or 10 mg/kg/day of atenolol was able to reduce SBP as compared to the hypertensive rats. Furthermore, pretreatment with CL for 14 days led to a significant inhibitory effect on ACE and arginase activities in the serum and kidney. In addition, CL increased NO level and decreased serum creatinine and urea levels.
With the same experimental protocol, Akinyemi et al. tested the activity of CL on platelets ectonucleotidase and adenosine deaminase (ADA) activities32, as well as inflammatory cytokines and enzyme activities of cholinergic and purinergic systems33. Intake of CL caused a reduction in adenosine triphosphate (ATP) hydrolysis and ADA activity but an elevation in adenosine diphosphate (ADP) and adenosine monophosphate (AMP) hydrolysis.32 Pretreatment with CL reduced the serum levels of cytokines involved in inflammation, namely interleukin (IL)-1, IL-6, tumour necrosis factor-α and interferon-γ, and increased in anti-inflammatory agent (IL-10) level.33 Akinyemi et al. reported that acetylcholinesterase activity in peripheral lymphocytes was significantly reduced by CL.33 Likewise, serum butyryl-cholinesterase activity was clearly decreased by CL in rats receiving L-NAME.33 Hydrolysis of ATP as well as ADP by ectonucleoside triphosphate phosphohydrolases (NTPDases) together with ADA activity in peripheral lymphocytes were also found to be reduced following the supplementation of CL.33
Li et al. shown that treatment with 10 mg/kg/day of demethoxycurcumin (DMC) for three weeks decreased the SBP in 30-week-old male SHR.34 In addition, long-term administration of DMC was able to diminish the increased endothelium-dependent contraction by ACh and enhance the reduced ACh-induced relaxation in the renal arteries of SHR. It was reported that phosphorylation of endothelium nitric oxide synthase (P-eNOS) and cyclooxygenase 2 (COX-2) protein expression were reduced and elevated, respectively, in renal arteries from SHR. Those changes were corrected by the treatment of DMC for three weeks. Therefore, DMC may have a crucial position in contributing to the improvement of endothelial function in hypertension by reducing COX-2 expression.
Xia et al. reported that curcumin treatment significantly decreased the BP of male albino rats in a dose (60 mg/kg/day and 120 mg/kg/day)- and time (14 days and 28 days)- dependent manner.35 The rats undergone surgical operation to produce cranial window. Curcumin significantly increased red blood cell velocity, microvascular diameter, vasomotion and number of open capillaries. On the other hand, circulating endothelial cells were reduced following curcumin administration. The obtained results suggested that curcumin was able to maintain endothelium integrity. Furthermore, by increasing the number of open capillaries, this in turn can reduce the peripheral resistance and eventually decrease the BP in rats receiving curcumin. The rise in vasomotion as in amplitude and frequency, led to an increase of blood flow, which may be useful for curcumin to regulate the cerebral microcirculation in hypertension.
Adult male 8-week old C57BI/6J mice were used by Yao et al. to examine the activity of curcumin on angiotensin II (Ang II)-induced hypertension.36 The animals were assigned to the control group and experimental groups receiving Ang II or Ang II with curcumin (300 mg/kg/day). After one week of treatment, curcumin was shown to reduce SBP and DBP in Ang II-treated mice. In addition, curcumin suppressed the contractions of mesenteric arteries in response to the increasing concentration of Ang II. Moreover, curcumin also decreased the expression of angiotensin II type 1 receptor (AT1R) protein expression in thoracic aorta of Ang II-induced hypertensive mice. Based on the obtained results, curcumin may exert its inhibitory effect on hypertension by reducing AT1R expression as well as to attenuate vasoconstriction elicited by Ang II.
Table 1: Effect of Curcuma longa (CL) and its constituents on blood pressure (BP) in animal model
| Author | Study Model | Constituent/Dose | Study Duration | BP Measurement | Finding |
| Goto et al.25 (2005) | 8-week-old male SHR | 3% w/w CL (p.o.) | 12 weeks | Tail-cuff | no effect on SBP |
| Adaramoye et al.26 (2009) | 8- to 10-week old male Wistar normotensive rats | Methanolic extract of CL (10, 20, 30 mg/kg) (i.v.) | NA | Intra-aortic:
lower abdominal aorta |
↓ MAP
(dose-dependent) |
| Hlavačková et al.27 (2011) | 12-week-old male Wistar rats treated with L-NAME (40 mg/kg/day) | curcumin 100 mg/kg/day (p.o.) | 6 weeks | Tail-cuff
|
↓ SBP
|
| Nakmareong et al.28 (2011) | male Sprague-Dawley rats treated with L-NAME (50 mg/kg/day) | curcumin
50 mg/kg/day, 100 mg/kg/day THC 50 mg/kg/day, 100 mg/kg/day (p.o.) |
3 weeks | Tail-cuff
Intra-arterial: left femoral artery
|
↓ SBP
↓ Arterial BP dose-dependently (SBP, DBP, MAP)
THC more effective than curcumin |
| Nakmareong et al.29 (2012) | male Sprague-Dawley rats treated with L-NAME (50 mg/kg/day) for 3 weeks | THC 50 mg/kg/day, 100 mg/kg/day for 2 weeks (i.g.) | 5 weeks | Tail-cuff
Intra-arterial: left femoral artery
|
↓ SBP
↓ Arterial BP dose-dependently (SBP, DBP, MAP) |
| Boonla et al.30 (2014) | 2K-1C male Sprague-Dawley rats | curcumin
50 mg/kg/day, 100 mg/kg/day (p.o.) |
6 weeks | Tail-cuff
Intra-arterial: femoral artery
|
↓ SBP (dose-dependent)
↓ Arterial BP dose-dependently (SBP, DBP, MAP) |
| Akinyemi et al.31-33 (2015; 2016a; 2016b) | male Wistar rats treated with L-NAME (40 mg/kg/day) for 10 days | turmeric aqueous extract 4% for 14 days (p.o.) | 24 days | Tail-cuff | ↓ SBP |
| Li et al.34 (2016) | 30-week-old male Wistar-Kyoto rats and SHR | demethoxycurcumin
10 mg/kg/day (i.p.) |
3 weeks | Tail-cuff | ↓ SBP in SHR |
| Xia et al.35 (2016) | adult male albino Wistar rats | curcumin
60 mg/kg/day 120 mg/kg/day (i.p.) |
4 weeks | Intra-arterial:
Carotid artery
Microvascular pressure: Servo-nulling pressure system in brain |
↓ arterial BP
(dose- and time-dependent) NA |
| Yao et al.36 (2016) | 8-week-old male C57BI/6J mice treated with Ang II (490 ng/min/kg) | curcumin
300 mg/kg/day (p.o.) |
1 week | Tail-cuff | ↓ SBP, DBP |
Symbol indicates: ↓, decrease
Abbreviations: 2K-1C, 2 kidney-1 clip; Ang II, angiotensin II; DBP, diastolic blood pressure; i.g., intragastric; i.p., intraperitoneal; i.v., intravenous; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; MAP, mean arterial pressure; NA, not applicable/available; p.o., per os; SBP, systolic blood pressure; SHR, spontaneously hypertensive rat; THC, tetrahydrocurcumin
Overall, animal studies in general show a protective effect of CL or its constituents on BP (Table 1). CL or its constituents can be used as an individual treatment to inhibit increased of BP in animals. Combination of curcumin and piperine has been evaluated for their preventive effects on BP. Piperine was shown to increase bioavailability of curcumin.37 Nevertheless, curcumin alone demonstrated a greater beneficial effect than co-administered with piperine in reducing BP.27 Hypertension is multifactorial in nature. The antihypertensive effects of a particular drug are not able to be fully explained by using one animal model. There are many pathways which responsible for the development of BP dysregulation. Therefore, various experimental models such as genetic hypertension, environmental hypertension, pharmacological hypertension and renal hypertension have been employed to evaluate the antihypertensive action of CL or its constituents. The dose of CL or its constituents used in the aforementioned studies varies greatly. The common administered doses are 50 mg/kg/day and 100 mg/kg/day. None of the studies reported any adverse reactions suffered by the laboratory animals.
The effects of CL on blood pressure in human studies
Thirty-nine healthy middle-aged and older adults with mostly Caucasians were divided into placebo (n = 19) and curcumin-supplemented groups (n = 20) for 12 weeks by Santos-Parker et al.38 Ingestion of 2000 mg/day of curcumin (Longvida® pill) improved endothelial function of resistance and conduit arteries in these healthy middle-aged and older adults. Intake of curcumin was shown to reverse the reduction in forearm blood flow following infusion of ACh in the presence of L-NAME, a NOS inhibitor. In addition, 12 weeks of curcumin reduced oxidative stress-mediated suppression of endothelium-dependent vasodilatation in response to co-administration of antioxidant vitamin C. The protective action may be due to the ability of curcumin to increase NO bioavailability and reduce oxidative stress. However, there was no difference in BP between placebo and curcumin-treated groups. The possible reason is curcumin has no hypotensive effect on normal healthy subjects.
Choi et al. (2018) analysed data using the Korean National Health and Nutrition Examination Survey (KNHANES) 2013 to investigate the effect of curry consumption in reducing hypertension.39 This cross-sectional study involving 1350 relatively healthy subjects were divided into two groups: 603 adults that had taken a curry dish more than once a month over the past year, and the remaining 747 adults had not. The most typical curry powder available in the market of South Korea is 10% of total 20 g portion for each person. This amount equivalent to about 2 g of CL with 1 mg to 11.5 mg of curcumin found in the curry powder.39 However, due to the nature of this study, detailed information regarding to the amount of curry powder intake was not known. Choi et al. revealed that subjects consumed curry regularly showed a reduced odd ratio for the prevalence of hypertension compared to those without consumed it.39 However, when age, sex, smoking and body mass index were adjusted, only the non-curry intake group showed significant odd ratio for the prevalence of hypertension. These findings indicated that the presence of other factors may have an impact on the relationship for those consumed curry.
Proposed Pharmacological Properties of CL against Hypertension
The precise pathway for CL to lower BP is not completely established. The antihypertensive effects of CL may be attributed to the presence of its active constituents, each with specific modes of action. Some potential mechanisms concerned with BP reduction are suggested, including antioxidant, anti-inflammation, Ca2+ concentration interference, β2-adrenergic receptor stimulation, and renin-angiotensin system inhibition (Figure 1).
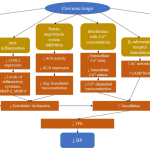 |
Figure 1: Proposed mechanisms of action responsible for reduced blood pressure (BP) following administration of Curcuma longa (CL) |
Symbols indicate: ↑, increase; ↓, decrease
Abbreviations: AC, adenylate cyclase; ACE, angiotensin-converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; Ca2+, calcium (II) ion; cAMP, cyclic adenosine monophosphate; COX, cyclooxygenase; MMP, matrix metalloproteinase; NO, nitric oxide; NOS, nitric oxide synthase; PKC, protein kinase C; TPR, total peripheral resistance
Oxidative stress takes place during a disturbed balance between the levels of antioxidant and pro-oxidant, which is often linked to the pathogenesis of hypertension. Impaired defence mechanism of antioxidant coupled together with over production of free radicals lead to cell damage. ROS such as superoxide will react with NO to generate peroxynitrite, a potent pro-oxidant. NO contributes to the maintenance of vascular homeostasis by causing vasodilatation, and reducing total peripheral resistance.
Curcumin is known for its strong antioxidant property. Methanolic extract of CL (200 mg/kg/day) was gavaged to the male Wistar rats prior to the administration of L-NAME for three weeks.40 Rats in L-NAME group showed a significant increase in serum urea, creatinine kinase and alanine aminotransferase levels. Furthermore, lipid peroxidation was augmented in the liver, heart and kidney isolated from the L-NAME-treated rats. Supplementation of CL caused a significant reduction of these biochemical indices in rats receiving L-NAME. Generation of lipid peroxidation products might be due to the reduced activities of enzymatic antioxidants as observed in this study. L-NAME greatly reduced the levels of hepatic catalase, superoxide dismutase (SOD), glutathione S-transferase, GSH as well as renal SOD and GSH. In contrast, pretreatment with CL restored the reduced antioxidant activities in L-NAME group to a level similar to the control that received only corn oil as a drug vehicle.
Lekshmi et al. assessed the effects of CL against cellular and low-density lipoprotein (LDL) cholesterol oxidation.41 In this study, fresh rhizomes of CL were extracted with hexane, ethyl acetate, methanol, and water, respectively. Ethyl acetate extract was demonstrated to exhibit the highest 2, 2’-diphenyl-1-picrylhydrazin (DPPH), 2, 2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), hydroxy and superoxide radical scavenging capacity compared to other tested extracts. In addition, ability of CL to reduce oxidative stress in cells was reported. Immortalised mouse myoblast cell line, C2C12 cells were used to induce oxidative stress using hydrogen peroxide. Ethyl acetate, methanol and water extracts showed a concentration-dependent reduction in cellular oxidative stress. The experimental concentration of 100 µg/mL of ethyl acetate was revealed to be comparable to the 25 µg/mL of ascorbic acid used as a positive control. Furthermore, ethyl acetate, methanol and water extracts were capable to inhibit LDL cholesterol oxidation in a concentration-dependent manner. Interestingly, ethyl acetate extract was reported to be 40 times more effective than ascorbic acid, an antioxidant. The capability of the extracts was suggested to be attributed to the presence of phenolics and curcuminoids.
ACE is the key enzyme converting Ang I to Ang II, a potent vasoconstrictor in regulating cardiovascular homeostasis. Oxidative stress is induced by Ang II via stimulation of nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase in addition to the generation of free radicas.42 Moreover, Ang II enhances lipid peroxidation43 and promotes synthesis of pro-oxidant cytokines,44,45 which cause a rise in the BP. Ang II has been reported to cause a reduction in the NOS expression.42 The Ang II-induced superoxide formation scavenges NO and reduces NO bioavailability.46 Consequently, this could lead to endothelial dysfunction.46
The antihypertensive activity of CL was examined by measuring its inhibitory activity on ACE by using hexane, ethyl acetate, methanol and water extracts.41 Ethyl acetate (IC50 = 0.06 µg/mL) extract had the highest ACE inhibitory capability followed by methanol (IC50 = 0.19 µg/mL) and water (IC50 = 0.38 µg/mL) extracts. However, hexane extract did not show any measurable inhibitory potential. All the three extracts (ethyl acetate, methanol and water) were more potent compared to captopril (IC50 = 6.28 µg/mL), an ACE inhibitor which is commonly prescribed for hypertensive patients.
Yao et al. investigated the effect of curcumin on AT1R expression using rat A10 vascular smooth muscle cell line.36 Curcumin was found to cause a concentration (10-5 M to 10-9 M)- and time (2 hours to 30 hours)-dependent decrease in AT1R protein expression. Furthermore, curcumin reduced messenger ribonucleic acid (mRNA) expression of AT1R upon examination using reverse transcription polymerase chain reaction (RT-PCR). Yao et al. proceeded the experiment by treating A10 cells with cycloheximide to inhibit translation step in protein synthesis. Actinomycin D was used to inhibit transcription as a measure of mRNA decay. Both of these drugs did not have inhibitory effect on curcumin in terms of its role in down-regulating the AT1R expression. Hence, the obtained results suggested that curcumin may down-regulate the expression of AT1R at the transcriptional level.
Human umbilical vein endothelial cells (HUVECs) were cultured and treated with Ang II for 12 hours with the presence or absence of demethoxycurcumin (DMC; 10 µmol/L).34 DMC is one of the major curcuminoids found in CL. Li et al. revealed that Ang II caused a reduction in NO generation in HUVECs by decreasing A23187 which served as a calcium ionophore to activate P-eNOS. Western blot assays demonstrated that down-regulation of P-eNOS expression in HUVECs treated with Ang II was to be reversed by the addition of DMC. Similar results were obtained with the addition of celecoxib, a COX-2 inhibitor or losartan, an AT1R blocker. Following the normalisation of P-eNOS expression, DMC, celecoxib and losartan were able to restore the NO production in HUVECs.
Nuclear factor kappa B (NF-κB) is a critical regulator for transcription by mediating inflammation, immune and stress responses. Furthermore, NF-κB involves in the signalling pathways of cell development, differentiation, proliferation and apoptosis. NF-κB is to be activated upon stimulation by pro-inflammatory cytokines. It is an inducible cell transcription factor and a key regulator in the production of COX-2. Previous studies have documented that CL was able to inhibit NF-κB pathway and resulted in a reduction of cytokines and COX-2 expression.47,48
Renal arteries from normotensive and hypertensive patients undergoing radical nephrectomy were obtained by Li et al.34 The arteries from hypertensive patients were cultured for 12 hours with or without DMC (10 µmol/L). Both Western blot assay and immunofluorescence microscopy revealed that the COX-2 expression was higher for hypertensive than normotensive patients. The increased COX-2 protein expression was to be depressed with the presence of DMC. Li et al. also conducted an in-vitro study to observe the effect of DMC on isolated renal arteries from SHR.34 The arteries exhibited a reduced endothelium-dependent relaxation in response to ACh. However, a greater contraction by ACh was observed when the arteries were pre-incubated with L-NAME which acted as an inhibitor of NOS. These altered vascular responses were not that severe in the normotensive Wistar-Kyoto rats. The renal arteries exposed to DMC (10 µmol/L) for 12 hours enhanced relaxation as well as suppressed contraction induced by ACh. Treatment with celecoxib also produced similar findings. The COX-2 protein expression was down-regulated following the exposure of DMC for 12 hours.
Moohammadaree et al. explored the vasorelaxant action of hexahydrocurcumin (HHC), a metabolite of curcumin, using isolated thoracic aorta from male Wistar rats.49 It was shown that cumulative concentration of HHC ranging from 1 nM to 1 mM attenuated the contraction elicited by PE or KCl. There was no difference of vasorelaxant response observed between the rings with undamaged endothelium and endothelium that had been rubbed off. The obtained findings may imply that HHC induced relaxation via an endothelium-independent mechanism by targeting smooth muscle cells on the vasculature. Ability of HHC to relax aortic rings precontracted with either PE or KCl may suggest an inhibition of extracellular Ca2+ influx. In addition. pre-incubation with HHC (0.1 µM to 100 µM) suppressed sustained contraction evoked by increasing concentration of CaCl2 and transient contraction induced by PE or caffeine in the absence of Ca2+. Therefore, HHC may exhibit inhibitory activity on Ca2+ mobilisation from internal stores.
Furthermore, increasing concentration of HHC (1 nM to 1 mM) was reported to abolish vascular contractility induced by phorbol 12-myristate 13-acetate (PMA), which stimulates protein kinase C (PKC).49 PKC has been demonstrated to mediate vascular contraction through direct phosphorylating myosin light chain kinase.50 Therefore, a Ca2+-independent mechanism may be proposed to be involved in the PKC-mediated contractile response. The obtained results demonstrated that the ability of HHC to evoke vasorelaxation may occur through a blockade of PKC. Aside from that, pretreatment with a non-selective β-adrenergic receptor blocker, propranolol, caused a reduction in vasorelaxation elicited by HHC. Stimulation of β2-adrenergic receptor produces an increased activity of adenylate cyclase, and followed by an increment in the intracellular concentration of cyclic adenosine monophosphate.51 When β2-adrenergic receptor is blocked by propranolol, HHC is not able to stimulate the receptor to produce vasorelaxation. Hence, HHC may produce vasorelaxant activity via an agonistic effect on β2-adrenergic receptor.
Conclusion
CL has been used as a traditional medicine for various ailment without causing further detrimental effects in human. Previously, CL has been documented to exert beneficial action on cancers in the clinical trials. Non-communicable disease is one of the causes with high mortality rate. Since hypertension is a risk factor of CVD, further well-designed studies should be performed to confirm the efficacy of CL on human population. This is a field for further research and development to incorporate CL with different antihypertensive medications to evaluate their potential additive and/or synergistic effects and desirable therapeutic properties in reducing BP. In addition, pharmacokinetic profiles of CL should be improved in view of its low oral bioavailability and rapid metabolism. This in turn can increase the product value and further expand its market in the pharmaceutical industry.
Acknowledgements
The author would like to thank Universiti Kebangsaan Malaysia for supporting the writing of this manuscript.
Conflict of Interest
The author declares no conflict of interest.
References
- Rapsomaniki E., Timmis A., George J., et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899-1911.
CrossRef - Whelton P. K., Carey R. M., Aronow W. S., et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018:71(6):1269-1324.
CrossRef - Forouzanfar M. H., Liu P., Roth G. A., et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017:317(2):165-182.
CrossRef - Wang Y. X., Song L., Xing A. J., et al. Predictive value of cumulative blood pressure for all-cause mortality and cardiovascular events. Sci. Rep. 2017;7:41969.
CrossRef - Reboussin D. M., Allen N. B., Griswold M. E., et al. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e116-e135.
CrossRef - Chobanian A.V., Bakris G. L., Black H. R., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
CrossRef - Prasad S., Gupta S. C., Tyagi A. K and Aggarwal B. B. Curcumin, a component of golden spice from bedside to bench and back. Biotechnol. Adv. 2014;32(6):1053-1064.
CrossRef - Gupta S. C., Patchva S and Aggarwal B. B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195-218.
CrossRef - Ak T and Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174(1):27-37.
CrossRef - González-Reyes S., Guzmán-Beltrán S., Medina-Campos O. N and Pedraza-Chaverri J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid. Med. Cell. Longev. 2013;2013: 801418.
- Bagad A. S., Joseph J. A., Bhaskaran N and Agarwal A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Adv. Pharmacol. Sci. 2013;2013:805756.
- Qabaha K., Abu-Lafi S and Al-Rimawi F. Anti-inflammatory activities of ethanolic extracts of Curcuma longa (turmeric) and cinnamon (Cinnamomum verum). J. Food Nutr. Res. .2017;5(9): 668-673.
- Gunes H., Gulen D., Mutlu R., Gumus A., Tas T and Topkaya A. E. Antibacterial effects of curcumin an in vitro minimum inhibitory concentration study. Toxicol. Ind. Health. 2013;32(2): 246-250.
CrossRef - Tyagi P., Singh M., Kumari H., Kumari A and Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10(3):e0121313.
CrossRef - Hu A., Huang J. J., Li R. L., et al. Curcumin as therapeutics for the treatment of head and neck squamous cell carcinoma by activating SIRT1. Sci. Rep. .2015;5:13429.
CrossRef - Koohpar Z. K., Entezari M., Movafagh A and Hashemi A. Anticancer activity of curcumin on human breast adenocarcinoma: role of Mcl-1 gene. Iran J. Cancer Prev. 2015;8(3):e2331.
CrossRef - Mohammed A., Wudil A. M., Alhassan A. J., Imam A. A., Muhammad I. U and Idi A. Hypoglycemic activity of Curcuma longa Linn root extracts on alloxan- induced diabetic rats. Saudi J. Life Sci. 2017;2(2):43-49.
- Kyung E. J., Kim H. B., Hwang E. S., et al. Evaluation of hepatoprotective effect of curcumin on liver cirrhosis using a combination of biochemical analysis and magnetic resonance-based electrical conductivity imaging. Mediators Inflamm. 2018;2018:5491797.
CrossRef - Li R., Xiang C., Ye M., Li H. F., Zhang X and Guo D.A. Qualitative and quantitative analysis of curcuminoids in herbal medicines derived from Curcuma species. Food Chem. 2011;126(4):1890-1895.
CrossRef - Inoue K., Nomura C., Ito S., Nagatsu A., Hino T and Oka H. Purification of curcumin, demethoxycurcumin, and bisdemethoxycurcumin by high-speed countercurrent chromatography. J. Agric. Food Chem. 2008;56(20):9328-9336.
CrossRef - Zhang J., Jinnai S., Ikeda R., Wada M., Hayashida S and Nakashima K. A simple HPLC-fluorescence method for quantitation of curcuminoids and its application to turmeric products. Anal. Sci. 2009;25(3):385-388.
CrossRef - Wang Y. J., Pan M. H., Cheng A. L., et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15(12):1867-1876.
CrossRef - Anand P., Kunnumakkara A. B., Newman R. A and Aggarwal B. B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807-818.
CrossRef - Kocher A., Schiborr C., Behnam D and Frank J. The oral bioavailability of curcuminoids in healthy humans is markedly enhanced by micellar solubilisation but not further improved by simultaneous ingestion of sesamin, ferulic acid, naringenin and xanthohumol. J. Funct. Foods. 2015;14:183-191.
CrossRef - Goto H., Sasaki Y., Fushimi H., Shibahara N., Shimada Y and Komatsu K. Effect of curcuma herbs on vasomotion and hemorheology in spontaneously hypertensive rat. Am. J. Chin. Med. 2005;33(3):449-457.
CrossRef - Adaramoye O. A., Anjos R. M., Almeida M. M., et al. Hypotensive and endothelium-independent vasorelaxant effects of methanolic extract from Curcuma longa L. in rats. J. Ethnopharmacol. 2009;124(3):457-462.
CrossRef - Hlavačková L., Janegová A., Uličná O., Janega P., Cerná A and Babál P. Spice up the hypertension diet – curcumin and piperine prevent remodeling of aorta in experimental L-NAME induced hypertension. Nutri. Metab. 2011;8:72.
CrossRef - Nakmareong S., Kukongviriyapan U., Pakdeechote P., et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011:383(5):519-529.
CrossRef - Nakmareong S., Kukongviriyapan U., Pakdeechote P., et al. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens. Res. 2012;35(4):418-425.
CrossRef - Boonla O., Kukongviriyapan U., Pakdeechote P., et al. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44-53.
CrossRef - Akinyemi A. J., Thomé G. R., Morsch V. M., et al. Effect of dietary supplementation of ginger and turmeric rhizomes on angiotensin-1 converting enzyme (ACE) and arginase activities in L-NAME induced hypertensive rats. J. Funct. Foods. 2015;17:792-801.
CrossRef - Akinyemi A. J., Thomé G. R., Morsch V. M., et al. Dietary supplementation of ginger and turmeric rhizomes modulates platelets ectonucleotidase and adenosine deaminase activities in normotensive and hypertensive rats. Phytother. Res. 2016;30(7):1156-1163.
CrossRef - Akinyemi A. J., Thomé G. R., Morsch V. M., et al. Effect of ginger and turmeric rhizomes on inflammatory cytokines levels and enzyme activities of cholinergic and purinergic systems in hypertensive rats. Planta Med. 2016;82(7):612-620.
CrossRef - Li Y., Tian D., Zhu C and Ren L. Demethoxycurcumin preserves renovascular function by downregulating COX-2 expression in hypertension. Oxid. Med. Cell. Longev. 2016;2016:9045736.
- Xia J., Wang H., Zhang Q. M., Zheng Z and Han Z. M. The therapeutic effect of curcumin in male albino rats and its putative mechanisms on cerebral microvascular flow. Brain Res. 2016;1642: 131-135.
CrossRef - Yao Y., Wang W., Li M., et al. Curcumin exerts its anti-hypertensive effect by down-regulating the AT1 receptor in vascular smooth muscle cells. Sci. Rep. 2016;6:25579.
CrossRef - Suresh D and Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010;131:682-691.
- Santos-Parker J. R., Strahler T. R., Bassett C. J., Bispham N. Z., Chonchol M. B and Seals D. R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9(1):187-208.
CrossRef - Choi J. W., Oh C., Shim S. Y., Jeong S., Kim H. S and Kim M. S. Reduction in prevalence of hypertension and blood heavy metals among curry-consumed Korean. Tohoku J. Exp. Med. 2018;244(3):219-229.
CrossRef - Adaramoye O. A., Nwosu I. O and Farombi E. O. Subacute effect of NG-nitro-I-arginine methyl-ester (L-NAME) on biochemical indices in rats: protective effects of Kolaviron and extract of Curcuma longa L. Pharmacognosy Res. 2012;4(3):127-133.
CrossRef - Lekshmi P. C., Arimboor R., Nisha V. M., Menon A. N and Raghu K. G. In vitro antidiabetic and inhibitory potential of turmeric (Curcuma longa L) rhizome against cellular and LDL oxidation and angiotensin converting enzyme. J. Food Sci. Technol. 2014;51(12):3910-3917.
CrossRef - Rajagopalan S., Kurz S., Münzel T., et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97(8):1916-1923.
CrossRef - Kedziora-Kornatowska K. Z., Luciak M and Paszkowski J. Lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney: effect of treatment with angiotensin convertase inhibitors. IUBMB Life. 2000;49(4):303-307.
CrossRef - Ruiz-Ortega M., Ruperez M., Lorenzo O., et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. Suppl. 2003;62(82):S12-S22.
- Cowling R. T., Zhang X., Reese V. C., et al. Effects of cytokine treatment on angiotensin II type 1A receptor transcription and splicing in rat cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2005;289(3):1176-1183.
CrossRef - Elmarakby A. A and Imig J. D. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin. Sci. 2010;118(4):291-301.
CrossRef - Gonzales A. M and Orlando R. A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008;5:17.
CrossRef - Olivera A., Moore T. W., Hu F., et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012;12(2):368-377.
CrossRef - Moohammadaree A., Changtam C., Wicha P., Suksamrarn A., Tocharus J and Tocharus C. Mechanisms of vasorelaxation induced by hexahydrocurcumin on isolated rat thoracic aorta. Phytother. Res. 2015;29(11):1806-1813.
CrossRef - Christova T., Duridanova D and Setchenska M. Protein kinase C and smooth muscle contraction. Biomed. Rev. 1997;8:87-100.
CrossRef - McGraw D. W and Liggett S. B. Molecular mechanisms of β2-adrenergic receptor function and regulation. Proc. Am. Thorac. Soc. 2005;2(4):292-296.
CrossRef








