Manuscript accepted on :17-May-2018
Published online on: --
Plagiarism Check: Yes
Figueroa-Valverde Lauro1, , Rosas-Nexticapa Marcela2
, Rosas-Nexticapa Marcela2 , Mateu-Armand Virginia2
, Mateu-Armand Virginia2 , Herrera-Meza Socorro3
, Herrera-Meza Socorro3 , Díaz-Cedillo Francisco4, García-Cervera Elodia1
, Díaz-Cedillo Francisco4, García-Cervera Elodia1 , Pool-Gómez Eduardo1
, Pool-Gómez Eduardo1 , García-Martínez Rolando1
, García-Martínez Rolando1 , Cauich-Carrillo Regina1
, Cauich-Carrillo Regina1 , Euan-Hau Saidy1, Parra-Galindo Perla2
, Euan-Hau Saidy1, Parra-Galindo Perla2
1Laboratory of Pharmaco-Chemistry, Faculty of Chemical Biological Sciences, University, University Autonomous of Campeche, Av. Agustín Melgar s/n, Col Buenavista C.P. 24039 Campeche, Camp.,México.
2Facultad de Nutrición, Universidad Veracruzana, Médicos y Odontologos s/n C.P. 91010, Unidad del Bosque Xalapa Veracruz, México.
3Instituto de Investigaciones Psicológicas, Universidad Veracruzana. Av. Dr. Luis Castelazo Ayala s/n Col Industrial Animas. C.P. 91190, Xalapa, Veracruz, México.
4 Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México.
Corresponding Author E-Mail: rosasnm@yahoo.com.mx
DOI : https://dx.doi.org/10.13005/bpj/1559
Abstract
The main objective of this study was to evaluate the biological activity of a new compound (derived from aza-bicyclo-carboxylic acid) against heart failure caused by the ischemia- reperfusion phenomenon. In addition, to characterize de molecular mechanism involved in the effect exerted by aza-bicyclo-carboxylic acid against infarction area, some drugs such as prazosin, metoprolol, propanolol, tamoxifen, flutamide, finasteride, nifedipine, levosimedan, adenosine, rolofylline, isoproterenol and the compound ZM-241385 were used as pharmacological tools. The data found indicated that biological activity induced by compound 3 on infarction area only was similar at effect exerted by adenosine; however, the effect produced by compound 3 was blocked with of rolofylline. Other data showed that the biological activity of compound 3 decreases the cAMP levels in a time-dependent manner. In conclusion, the results indicate that compound 3 can produce a cardioprotective effect against myocardial ischemia-reperfusion injury translated as a decrease on infarction area; this phenomenon involves A1-adenosine receptor activation and, as a result may cause changes in cAMP levels.
Keywords
Adenosine; Camp; Ischemia; Reperfusion; Rolofylline
Download this article as:| Copy the following to cite this article: Lauro F. V, Marcela R. N, Virginia M. A, Socorro H. M, Francisco D. C, Elodia G. C, Eduardo P. G, Rolando G. M, Regina C. C, Saidy E. H, Perla P. G. Evaluation of Biological Activity Exerted by an Aza-Bicyclo-Carboxylic Acid Derivative Using an Ischemia-Reperfusion Injury Model. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Lauro F. V, Marcela R. N, Virginia M. A, Socorro H. M, Francisco D. C, Elodia G. C, Eduardo P. G, Rolando G. M, Regina C. C, Saidy E. H, Perla P. G. Evaluation of Biological Activity Exerted by an Aza-Bicyclo-Carboxylic Acid Derivative Using an Ischemia-Reperfusion Injury Model. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24759 |
Introduction
Heart failure is the main cause of death in patients with heart disease1-3; this phenomenon is due to the cardiac myocyte cell death caused by prolonged myocardial ischemia that can be translated as myocardial infarction, which consequently results in some changes in the cardiac work of both ventricles.4,5 Several reports suggest that restoration of blood flow in some case can decrease the cardiac necrosis; nevertheless, other studies indicate that the reperfusion may increase the tissue injury.6 It is important to mention, that there are several drugs such as the Cyclosporin-A which showed biological activity against ischemia-reperfusion injury in an animal model.7 Other data indicate that rapamycin which can exert benefic effects on myocardial infarction in isolated mouse heart via mitochondrial potassium channel activation.8 In addition, a study showed that glibenclamide reduces myocardial damage in ischemia-reperfusion model through potassium channel activation.9 Also, a study showed that a benzenamine-derivative (SEA0400) can decrease the reperfusion injury using some “in vitro” and “in vivo” models.10 Recently, a study showed that a naphthalene-prazosin derivative exerts a cardioprotective effect against myocardial necrosis after ischemia-reperfusion injury via the calcium channels activation.11 All these results indicate that some compounds may exert effects on reperfusion-injury; however, the cell site where they produce their biological activity is not very clear. To characterize this phenomenon, the biological activity exerted by an aza-bicyclo-carboxylic acid derivative was evaluated using an ischemia-reperfusion injury model.
Material and Methods
General methods.
The compounds 3-(2-Amino-ethyl)-1,5-dinitro-9-(3-oxo-butyl)-3-aza-bicyclo [3.3.1]non-6-ene-7-carboxylic acid (1) and (2-Acetyl-9a-methyl-7-oxo-dodecahydro-cyclopenta[a] naphthalen-8-yl)-acetic acid (2) were prepared using a previously methods reported.12,13 All chemicals were analytical grade supplied by Sigma-Aldrich Co. Ltd. Elemental analysis was performed with an elemental analyzer (Perkin Elmer Ser. II CHNS/0 2400). Infrared spectra were recorded with a FTR iS50 apparatus. Mass spectra were recorded with a Finnigan Trace GCPolaris Q. NMR spectra were obtained using a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz in CDCl3.
Chemical synthesis
Synthesis of 3-{2-[2-(2-Acetyl-9a-methyl-7-oxo-dodecahydro-cyclopenta[a]naphtha- lene-8-yl)-acetylamino]-ethyl}-1,5-dinitro-9-(3-oxo-butyl)-3-aza-bicyclo[3.3.1]non-6-ene-7-carboxylic acid (3)
In a round bottom flask (10 ml), the compound 1 (200 mg, 0.55 mmol), compound 2 (60 µl, 0.90 mmol), boric acid (50 mg, 0.80 mmol) and 5 ml of methanol were placed. After, the mixture was stirred for 72 h to room temperature. The reaction product was dried under reduced pressure and purified by crystallization using the methanol:water (4:1) system.
Biological activity
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal care and use committee of Autonomous University of Campeche (Faculty of Chemical-Biological Sciences) with No. PI-420/12 and were in accordance with the Guide for the Care and Use of Laboratory Animals.14 Male Wistar rats; weighing 200-250 g were obtained from Pharmacochemistry Laboratory of University Autonomous of Campeche (Faculty of Chemical-Biological Sciences).
Reagents
It is noteworthy, that drugs involved in this study were dissolved in methanol and from this solution all dilutions were obtained using a Krebs-Henseleit* solution (v/v).
Experimental Design I
Animals were anesthetized with pentobarbital (50 mg/Kg body weight) via intraperitoneal administration. After the animal was opened by a thoracic abdominal laparotomy and the heart was perfused via retrograde with the Krebs-Henseleit solution through a non-circulating perfusion system with a constant flow rate. It is important that the study population involved in each group was n = 9.
*Krebs-Henseleit solution (pH = 7.4, 37°C) composed by following system; 117.8 NaCl; 6 KCl; 1.75 CaCl2; 1.2 NaH2PO4; 1.2 MgSO4; 24.2 NaHCO3; 5 glucose and 5 sodium pyruvate (mmol). The solution was then bubbled with a mixture of O2/CO2 (95:5/5 %). The coronary flow (10 ml/min) was adjusted with a peristaltic pump and a period of equilibration was carried out for 15 min.
Ischemia-Reperfusion model
It is important to mention that after the equilibrium time; the hearts underwent a period of ischemia for 40 minutes due to closure of the perfusion system such as indicate some reports15 in absence (control) or presence of each of drug involved in this study (see design experimental). Following, the system was restarted and the hearts were reperfused by other 40 minutes with Krebs-Henseleit solution.
Histological Analysis
For histological evaluation, the technique modifies reported by Engelhardt16 was used. Cross sections from the heart were fixed with 10% paraformaldehyde for 8 h. Then, the samples are placed in a histocasette (Leica Mod. TP1020 SN: 042231418), for 12 h to be processed. After, the sections of heart were placed in a Paraffin Embedding Center (Leica EG1160 model) to form paraffin blocks which are cut into 2 µm slices using an aparatus Leica 50138178 model and following were introduced to bath water (Riossa-Rocha B7 SN: 070909 model). Then, the samples were placed on a slide, which was dried at 60 oC for 30 minutes in a Binder-ED23 apparatus. After, a solution of ethanol:xylol (1: 1) was added to the slides for cell clearance; then of 10 minutes, was washed with distilled water. To observe the tissue morphology, hematoxylin is added to the sample (for 1 minute), after which time it is washed again. Then eosin is added for 1 minute. Finally, ethanol/xylol (1:1) is added to the sample and the tissue is observed under the microscope. For morphometrical analysis, photographs of 20 ventricular sections were taken at 3320 magnifications (ZeissIM-35).
Biological Evaluation
Step I
Effects induced by the compound 1 against infarct area
The effect induced by the compound 1 on ischemia-reperfusion injury (translated as infarction area) was evaluated at dose of 0.001-100 nM.
After of each experiment the hearts were cut into two sections at right angles to the vertical axis. It is important to mention that the areas of the normal left ventricle non-risk region, area at risk, and infarct region were evaluated using a previously reported method.17,18
Biological activity produced by the compounds 1, 2 and 3 against infarct area
The effect induced by the compounds 1, 2 and 3 (at a dose of 0.001 nM) on the ischemia-reperfusion injury (translated as infarction area) was determined.
Step II
The biological activity induced by compound 3 on ischemia-reperfusion injury translated as infarction area was compared with effect exerted by different drugs involved in this study. In addition, it noteworthy that different doses administered in this study of each drug used as pharmacological tools have been previously reported17-27.
Evaluation of biological activity of the compound 3 against infarction area via sex hormons
The effect induced by the compounds 3 (0.001 nMm/min) or tamoxifen(1 nMm/min) or finasteride (nMm/min) or flutamide (nMm/min) on the ischemia-reperfusion injury (translated as infarction area) was determined.
Determination of biological effect of the compound 3 against infarction area through of adrenergic system
The effect exerted by the compounds 3 (0.001 nM) or prazosin (nMm/min), or propanolol (nMm/min) or metoprolol (nMm/min), on the ischemia-reperfusion injury (translated as infarction area) was asses.
Evaluation of biological activity of the compound 3 against infarction area via calcium channels
The effect induced by the compounds 3 (0.001 nM) or nifedipine (10 nM/min) or levosimedan (0.1 μM/min) on the ischemia-reperfusion injury (translated as infarction area) was determined.
Determination of biological effect of the compound 3 against infarction area through phosphodiesterase inhibition
The effect exerted by the compounds 3 (0.001 nM) or milrinone (0.025 μM/min), on the ischemia-reperfusion injury (translated as infarction area) was asses.
Evaluation of biological activity of the compound 3 against infarction area via ATP-ase inhibition
The effect induced by the compounds 3 (0.001 nM) or digoxine (20 μM/min) on the ischemia-reperfusion injury (translated as infarction area) was determined.
Determination of biological effect of the compound 3 against infarction area through purinergic system
The effect exerted by the compounds 3 (0.001 nM) or adenosine (140μg/min), on the ischemia-reperfusion injury (translated as infarction area) was asses.
Step III
Biological activity exerted of compound 3 against infarct area via adenosine receptors
The compound 3 was perfused (1 nM/min) in the hearts and infarct area was evaluated; then this experiment was repeated in presence of rolofylline (1 µM)28 or ZM- 241385 (1 ´ 10-7 M/min).29
Step IV
Effect exerted of Compound 3 on cAMP concentration
Isoproterenol (100 nM / min) was perfused on the heart for 3, 6, 9, 12 or 18 minutes and its effect on cAMP levels was determined using a previously reported method.39,31 After, the results obtained were compared with the biological activity exerted by the compound 3 (1 nM/min) and control on the concentration of cAMP.
Statistical analysis
The results were expressed as average ± SE, using each heart (n = 9) as its own control. In addition, the results were analyzed via Analysis of Variance using the SPSS 12.0 program.32 The differences in the values found were determinates with p = 0.05.
Results
Preparation of compound 3
Aza-bicyclo-carboxylic acid derivative (compound 3) was prepared by the reaction of 1 with 2 in presence of boric acid; yielding 75% of product (Figure 1) (melting point of 90-92°C). The infrared spectrum involved in compound 3 showed several signals at 1712, 1705, 1630 and 1320 (Vmax, cm-1). Additionally, the 1H NMR (Figure 1 and Table 1) and 13 C NMR (Table 2) for the compound 3 are showed down. Finally, the results of mass spectroscopy (MS) (70 ev) shown; m/z 658.32. Theoretical elementary analysis for the compound 3 (C33H46N4O10) showed the following results; C, 60.17; H, 7.04; N, 8.51; 0, 24.29; and experimental elementary analysis found was C, 60.08; H, 7.00.
![Figure 1: Synthesis of an aza-bicyclo-carboxylic acid (3). In the scheme A is shown the reaction of amino-bicyclo derivative (1) with the compound (2-Acetyl-9a-methyl-7-oxo-dodecahydro-cyclopenta[a]naphthalen-8-yl)-acetic acid (2) to form 3 using boric acid as catalyst (i). In addition, the 1H NMR (scheme B) showed two signals characteristic for the compound 3 at 0.90 and 2.10 ppm for methyl groups; at 10.40 ppm for both amide and hydroxyl groups.](https://biomedpharmajournal.org/wp-content/uploads/2018/12/Vol11No4_Eva_Fig_Fig1-150x150.jpg) |
Figure 1: Synthesis of an aza-bicyclo-carboxylic acid (3). In the scheme A is shown the reaction of amino-bicyclo derivative (1) with the compound (2-Acetyl-9a-methyl-7-oxo-dodecahydro-cyclopenta[a]naphthalen-8-yl)-acetic acid (2) to form 3 using boric acid as catalyst (i). In addition, the 1H NMR (scheme B) showed two signals characteristic for the compound 3 at 0.90 and 2.10 ppm for methyl groups; at 10.40 ppm for both amide and hydroxyl groups. |
Table 1: Spectra data of 1H NMR (proton nuclear magnetic resonance; 300 MHz, CDCl3) for the compound 3.
| δH: 0.90 (s, 3H), 1.34-1.56 (m, 8H), 1.66 (m, 1H), 1.74-1.80 (m, 2H), 1.88 (m, 1H), 1.94-2-04 (m, 2H), 2.10 (s, 6H), 2.16 (m, 1H), 2.20 (m, 1H), 2.32 (m, 1H), 2.34 (m, 1H), 2.50-2.70 (m, 2H), 3.04 (m, 1H), 3.06 (m, 2H), 3.18 (m, 4H), 3.19-3.52 (m, 2H), 3.66 (m, 1H), 3.86-4.22 (m, 3H), 8.46 (d, 1H, J = 2.08 Hz), 10.40 (broad, 2H) ppm. |
Biological activity
Biological activity induced by the compound 3 against ischemia injury at dose of 0.001-100 nM
The data indicated that compound 3 significantly decreased (p = 0.05) infarct size (percentage of area at risk) in a dose-dependent manner in comparison with the control (Figure 2).
![Figure 2: Effect exerted by the compound 3. The experimental data found shown that the compound 3 significantly decreased infarct size (expressed as a percentage of the area at risk) in a dose-dependent manner compared with the vehicle-treated hearts. The values indicate the mean ± S.E. of 9 experiments. D1 [0.001 nM]; D2 [0.01 nM]; D3 [0.1 nM]; D4 [1 nM]; D5 [10 nM] D6 [100 nM].](https://biomedpharmajournal.org/wp-content/uploads/2018/12/Vol11No4_Eva_Fig_Fig2-150x150.jpg) |
Figure 2: Effect exerted by the compound 3. The experimental data found shown that the compound 3 significantly decreased infarct size (expressed as a percentage of the area at risk) in a dose-dependent manner compared with the vehicle-treated hearts. The values indicate the mean ± S.E. of 9 experiments. D1 [0.001 nM]; D2 [0.01 nM]; D3 [0.1 nM]; D4 [1 nM]; D5 [10 nM] D6 [100 nM]. |
Histological issue evaluation
In the Figures 3 and 4 are shown a marked disruption of the myocardial structure characterized by appearance of extensive necrosis in conditions control; however this is lower in presence of compound 3.
Effects exerted by the compound 1-3
The biological activity induced by the compounds 1, 2 and 3 at a dose of 0.001 nM/min on infarct area were evaluated using an ischemia injury model. The experimental data found shown that compound 3 significantly decreases (p = 0.05) the infarct area compared with compounds 1, 2 and control conditions (Figure 5).
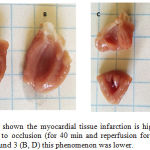 |
Figure 3: The scheme shown the myocardial tissue infarction is higher in vehicle (A, C) treated rats subjected to occlusion (for 40 min and reperfusion for 40 min); however, in presence of the compound 3 (B, D) this phenomenon was lower. |
Effects induced by the compound 3 against ischemia-reperfusion injury via sex hormones
The effect exerted by the compounds 1, 2, 3 and control conditions on infarct area were evaluated using an ischemia-reperfusion injury model (Figure 6). The results shown that compound 3 [0.001 nM/min] exert different biological activity (p = 0.05) compared with finasteride or tamoxifen or flutamide at dose of 1 nM.
![Figure 4: Histological evaluation of effect exerted by compound 3 against ischemia-reperfusion using the technique modifies reported by Engelhardt [28]. In the scheme E (control) a marked alteration of the structure of the myocardium section characterized by the appearance of extensive necrosis, bands of contraction and thinning of myofibrils were observed. In addition, in the scheme F is shown a decreased of myocardial necrosis by presence of compound 3.](https://biomedpharmajournal.org/wp-content/uploads/2018/12/Vol11No4_Eva_Fig_Fig4-150x150.jpg) |
Figure 4: Histological evaluation of effect exerted by compound 3 against ischemia-reperfusion using the technique modifies reported by Engelhardt [28]. In the scheme E (control) a marked alteration of the structure of the myocardium section characterized by the appearance of extensive necrosis, bands of contraction and thinning of myofibrils were observed. In addition, in the scheme F is shown a decreased of myocardial necrosis by presence of compound 3. |
Effect induced by the compound 3 against ischemia-reperfusion injury via adrenergic system
The results showed in the Figure 7, indicated that biological activity exerted by the compound 3 at dose of 0.001 nM significantly decrease (p = 0.05) the infarct area compared with prazosin, propanolol or metoprolol at dose of [1 nM/min].
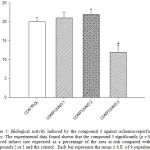 |
Figure 5: Biological activity induced by the compound 3 against ischemia-reperfusion injury. The experimental data found shown that the compound 3 significantly (p = 0.05) reduced infarct size expressed as a percentage of the area at risk compared with the compounds 2 or 3 and the control. Each bar represents the mean ± S.E. of 9 experiments. |
Effect produced by the compound 3, nifedipine and levosimedan on ischemia-reperfusion injury
In the Figure 7 is shown the biological activity of compound 3 [1nM], nifedipine [10 nM/min] and levosimedan [0.1µM/min] against ischemia-reperfusion injury. The experimental data found indicated that effect exerted by 3 was lower (p = 0.05) compared with nifedipine and levosimedan.
Biological activity induced by the compound 3, milrinone, digoxine and adenosine against ischemia-reperfusion injury
The experimental data (Figure 8) shown that effect induced by the compound 3 [0.001 nM] was lower (p = 0.05) compared with milrinone [0.025 mM/min]. However this effect was in a similar manner to biological activity exerted by adenosine [140μg/min] on infarct area.
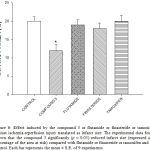 |
Figure 6: Effect induced by the compound 3 or flutamide or finasteride or tamoxifen against ischemia-reperfusion injury translated as infarct size. The experimental data found shown that the compound 3 significantly (p = 0.05) reduced infarct size (expressed as a percentage of the area at risk) compared with flutamide or finasteride or tamoxifen and the control. Each bar represents the mean ± S.E. of 9 experiments. |
Biological activity induced by the compound 3 in presence or absence of rolofylline or ZM-241385
The results (Figure 9) showed that compound 3 decrease the infarct area at dose of 1 nM; however, this phenomenon was significantly inhibited (p = 0.05) by rolofylline [1 ´ 10-7 M].
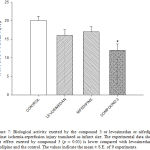 |
Figure 7: Biological activity exerted by the compound 3 or levosimedan or nifedipine against ischemia-reperfusion injury translated as infarct size. The experimental data shown that effect exerted by compound 3 (p = 0.05) is lower compared with levosimedan or nifedipine and the control. The values indicate the mean ± S.E. of 9 experiments. |
Biological activity of compound 3 and isoproterenol on the cAMP levels
The experimental data (Figure 10) shown that effect exerted by the compound 3 [1 nM] was lower (p = 0.05) compared with isoproterenol [100 nM] and conditions control.
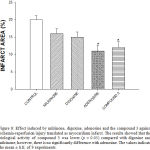 |
Figure 8: Effect induced by milrinone, digoxine, adenosine and the compound 3 against ischemia-reperfusion injury translated as myocardium infarct. The results showed that the biological activity of compound 3 was lower (p = 0.05) compared with digoxine and milrinone; however, there is no significantly difference with adenosine. The values indicate the mean ± S.E. of 9 experiments. |
Discussion
Several drugs have been used for treatment of ischemia-reperfusion such as propofol,33 methylene blue,34 resveratrol,35 rapamycin,36 nitro-derivative37 and others; however, each drug exerts its biological activity on the ischemia-reperfusion injury, through of several molecular mechanisms; this phenomenon could be the result of its different chemical characteristics. Therefore, in this study was prepared a new drug (aza-bicyclo-carboxylic acid derivative) to evaluate their biological activity against ischemia-reperfusion injury.
Chemical synthesis
There are several methods for preparation of azabicyclic; however, some reagents used require special conditions38-40; analyzing this data, in this report a new azabicyclic derivative was prepared using different chemical strategies. It is noteworthy, that in this reaction an amino-bicyclo (compound 1) was bound to the carboxyl group of oxo-naphthalene-acetic acid (compound 2) to form the compound 3 (C33H46N4O10) using boric acid as catalyst (Figure 1). Chemical structure of compound 3 was determinate using NMR spectroscopy (Table 1 and Table 2). The 1H NMR spectrum of the compound 3 showed signals at 0.90 ppm for methyl bound to dodecahydro-cyclopenta[a]naphthalene system; at 2.10 ppm for methyl bound to ketone groups; at 1.66, 1.88, 3.04-3.06 and 3.66 ppm for methylene groups involved in the arm bound to bicyclic ring; at 2.20 and 2.34 ppm for methylene group bound to both to dodecahydro-cyclopenta[a]naphthalene system and amide group; at 3.04, 3.19-3.52, 3.86-8.46 ppm for bicycle ring; at 3.18 ppm for arm bound to both amino and amide groups; at 10.40 ppm for both amide and hydroxyl groups. The 13 C NMR spectra displays chemical shifts at 14.22 ppm for methyl group bound to dodecahydro-cyclopenta[a]naphthalene system; at 27.86-29.60 ppm for methyl group bound to both ketone groups; at 18.90, 40.80, 56.72 and 59.08 ppm for methylene groups involved in the arm bound to bicyclic ring; at 34.48, 58.62, 93.04-142.10 ppm for bicycle ring; at 38.49 ppm for methylene group bound to both dodecahydro-cyclo- penta[a]naphthalene system and amide group; at 42.74 and 50.08 ppm for arm bound to both amino and amide groups; 167.72 ppm for carboxyl group; at 175.90-210.70 ppm for ketone groups. Finally, mass spectrum of compound 3 showed a molecular ion at m/z 658.32.
Table 2: Spectra data of 13C NMR (proton nuclear magnetic resonance; 300 MHz, CDCl3) for the compound 3.
| δC: 14.22, 18.90, 26.82, 27.86, 29.60, 30.52, 31.11, 34.35, 35.32, 38.48, 38.49, 39.68, 40.80, 41.65, 42.74, 43.72, 46.98, 47.15, 50.08, 51.82, 53.75, 56.72, 58.82, 59.08, 93.04, 98.13, 128.08, 142.10, 167.72, 175.90, 209.06, 209.58, 210.70 ppm. |
Biological activity
Effect induced by the compound 3 against ischemia injury.
In this study, biological activity induced by the compound 3 to different doses against infarct area were evaluated using an ischemia-reperfusion injury model. The experimental data found shown that compound 3 decreased the infarct area in a dose-dependent manner. Analyzing these results, other experiments were also carried out, in which the biological activity of compounds 1 and 2 was evaluated, to rule out the possibility that any of these compounds could exert some effect on the ischemia-reperfusion injury. The results showed that compound 3 significantly decrease the infarct area compared with compounds 1, 2 and control conditions (Figure 2 and 3); this phenomenon, indicated that the different functional groups involved in the chemical structure of 3 are the responsible for their biological activity against the ischemia-reperfusion injury translated as a reduction on infarction area.
 |
Figure 9: Biological activity exerted by the compound 3 against ischemia-reperfusion injury translated as myocardium infarct in absence or presence of rolofylline and ZM- 241385. The experimental data found shown that the compound 3 significantly (p = 0.05) reduced infarct size (expressed as a percentage of the area at risk); however, this phenomenon was inhibited in presence of rolofylline. The values indicate the mean ± S.E. of 9 experiments. |
 |
Figure 10: Biological activity induced by the compound 3 and isoproterenol on cAMP levels through of time. The experimental data found shown that cAMP levels was lower (p = 0.05) in the presence of the compound 3 compared with isoproterenol (3-18 min) and the control. The values indicate the mean ± S.E. of 6 experiments. |
Characterization of molecular mechanism involved in the biological activity induced by the compound 3 against ischemia-injury.
Analyzing data above mentioned and other reports which suggest that some drugs can produce effects on ischemia injury via activation of 5-alpha-reductase41 or interaction with both androgens and estrogens receptors42,43; in this study, the effect exerted by finasteride (5-alpha-reductase inhibitor) or tamoxifen (estrogen receptor antagonist) or flutamide (androgen receptor inhibitor) against ischemia-reperfusion injury was determinate and compared with the effect exerted by compound 3. The results shown that compound 3 exert different biological activity compared with finasteride or tamoxifen or flutamide (Figure 4); these results suggest that molecular mechanism involved in the effect induced by the compound 3 on ischemia-reperfusion injury was not via sex hormones activation.
On the other hand, also, other reports which suggest that ischemia-reperfusion injury may be reduced via adrenergic system activation44-46 were analyzed. Therefore, in this experimental study, the pharmacological activity exerted by prazosin or propranolol or metoprolol on ischemia injury was evaluated and compared with effect produce by compound 3 against ischemia-reperfusion injury. The data found shown that infarct area was lower in presence of compound 3 in comparison with prazosin, propanolol or metoprolol; this data suggest that effect exerted by the compound 3 on ischemia-reperfusion injury was not via a1 or b1 receptors activation.
Analyzing these data, and other studies which suggest that some drugs can induce effects against ischemia-reperfusion injury via calcium channel activation47,48; in this experimental investigation, the pharmacological activity induced by nifedipine (calcium antagonist) or levosimedan (calcium sensitizer)49 on ischemia-reperfusion injury was evaluated and compared with the biological activity induced by compound 3. The experimental results found shown that effect exerted by 3 was lower in comparison with nifedipine and levosimedan; this data indicated that molecular mechanism involved by 3 was not via calcium channels activation.
On The other hand, in this study was also validated the effect exerted by milrinone (phosphodiesterase inhibitor III)50 on myocardial infarct size to compare with biological activity exerted by compound 3. The experimental data indicated that compound 3 decreased infarct area was lower in comparison with biological activity of milrinone; these data indicated that molecular involved in the effect exerted by the compound 3 was not through of inhibition or activation of some phosphodiesterase.
In the search for the possible molecular mechanism involved in the effect exerted by compound 3, other reports were also analyzed; these studies indicate that ischemia-reperfusion in the subepicardial regions of ischemic tissue can be reduced in the presence of digoxin.51 Therefore, the effect produced by digoxin on ischemia-reperfusion injury was determined and compared with the effect exerted by the compound 3. The experimental data found shown that digoxin decreased the infarct area in comparison with control conditions; however, it is noteworthy that this effect was different compared with the compound 3; this results indicated that molecular mechanism was not via Na+/K+-ATPase inhibition.
Finally, other experiments were carried out to characterize the mechanism by which compound 3 induces its effect against ischemia-reperfusion injury. In this sense, the effect exerted by adenosine on the ischemia-reperfusion injury was evaluated and compared with the biological activity exerted by compound 3. The experimental data showed that the infarct area decreased in a similar way to the biological activity induced by compound 3. These results suggest that molecular mechanism could involve adenosine receptors52; to check this hypothesis; the effect exerted by compound 3 against ischemia-reperfusion injury was evaluated in presence of rolofylline (A1-adenosine receptor antagonist)53 or ZM-241385 (A2-adenosine receptor inhibitor).54 The experimental data found shown that biological activity exerted by the compound was only inhibited in presence of rolofylline; this results indicated that the molecular mechanism involved in the biological activity exerted by the compound 3 against ischemia-reperfusion injury which is translated as a decrease in the area of infarction was via A1-receptor activation; however, it is noteworthy that there are other reports which indicate that A1-Adenosine receptor activation can induce changes in cAMP levels55. Therefore, also the effect exerted by compound 3 on cAMP concentration was evaluated using isoproterenol as control. The experimental data found shown that compound 3 decreased the cAMP levels (3-18 min) in comparison with isoproterenol and control conditions. These results suggest that effect exerted by the compound 3 against ischemia-reperfusion injury translated as decreased of infarct area involves A1-adenosine receptor activation and consequently brings changes of cAMP levels.
Conclusions
The results indicate that compound 3 can produce a cardioprotective effect against myocardial ischemia-reperfusion injury translated as a decrease on infarction area; this phenomenon involves A1-adenosine receptor activation and, as a result may cause changes in cAMP levels.
Acknowledgement
None
Conflict of interest
The authors declare that they have no conflict of interest with any institutions.
References
- Cooper R., Cutler J., Desvigne P., Fortmann S., Friedman L., Havlik R. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137-3147.
CrossRef - Yusuf S., Reddy S., Ôunpuu S., Anand S. Global Burden of Cardiovascular Diseases: Part I: General Considerations, the Epidemiologic Transition Risk Factors and Impact of Urbanization. Circ. J. Am. Heart. Assoc. 2001;104:2746-2753.
- Ford E., Capewell S. Coronary Heart Disease Mortality Among Young Adults in the U.S. From 1980 Through 2002. J. Am. Coll. Cardiol. 2007;50:2128-2131.
CrossRef - Sarabia-Alcocer B., Figueroa-Valverde L., Díaz-Cedillo F., Hau-Heredia L., Rosas-Nexticapa M., García-Cervera E., Pool-Gómez E., García-Martínez R., Zepeda-Acosta B. Activity Induced by a Naphthalene-Prazosin Derivative on Ischemia Reperfusion Injury in Rats. Pharmacol. Pharm. 2014;5:130-142.
CrossRef - Hanlon P., Fu P., Wright G., Steenbergen C., Arcasoy M., Murphy E. Mechanisms of erythropoietin-mediated cardioprotection during ischemia-reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. FASEB J. 2005;19(10):1323-1325.
CrossRef - Klone R., Przyklener K., Whittaker P. Deleterious effects of oxygen radicals in ischemia reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115-1127.
CrossRef - Griffiths E., Halestrap A. Protection by Cyclosporin A of Ischemia/Reperfusion-Induced Damage in Isolated Rat Hearts. J. Mol. Cell. Cardiol. 1993;25:1461-1469.
CrossRef - Khan S., Salloum F., Das A., Xi L., Vetrovec G. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J. Mol. Cell. Cardiol. 2006;41:256-254.
CrossRef - Cole W., McPherson C., Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ. Res. 1991;69:571-581.
CrossRef - Takahashi K., Takahashi T., Suzuki T., Onishi M., Tanaka Y., Hamano A., et al. Protective effects of SEA0400, a novel and selective inhibitor of the Na+/Ca2+ exchanger on myocardial ischemia–reperfusion injuries. Eur. J. Pharmacol. 2003;458:155-162.
CrossRef - Sarabia-Alcocer B., Figueroa-Valverde L., Díaz-Cedillo F., Hau-Heredia L., Rosas-Nexticapa M., García-Cervera., et al. Activity Induced by a Naphthalene-Prazosin Derivative on Ischemia Reperfusion Injury in Rats. Pharmacol. Pharm. 2014;5:1130-1142.
CrossRef - Figueroa-Valverde L., Hau-Heredia L., García-Martinez R., López-Ramos M., Rosas-Nexticapa M., Herrera-Meza S., et al. Preparation of a hexynloxy-diazecin-naphtho-oxadiazocine derivative. Theoretical analysis of its interaction with the μ, k and d opioid-receptors. Biointer. Res. Appl. Chem. 2017;7(6):2243-2248.
- Figueroa-Valverde L., Hau-Heredia L., Rosas-Nexticapa M., Mateu-Armad M. V., Herrera-Meza S., Díaz-Cedillo F.,García-Cervera E., PoolGómez E., López-Ramos M. Preparation of three diazocin-derivatives via disconecction A-ring from progesterone. Bull. Environ. Pharmacol. Life Sci. 2018. (In press).
- Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. Am. Physiol. Soc. Physiologist. 1996;39:208-211.
- Figueroa-Valverde L., Díaz-Cedillo F., García-Cervera E., Rosas-Nexticapa M., Pool-Gómez E., Lopéz-Ramos M., et al. Evaluation of activity of an estrogen-derivative as cardioprotector drug using an ischemia-reperfusion injury model. Int. J. Clin. Exp. Med. 2015;8:12041-12055.
- Engelhardt S., Hein l., Wiesmann F., Lohse M. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. U S A. 1999;96:7059-7064.
CrossRef - Figueroa-Valverde L., Díaz-Cedillo F., García-Cervera E., Pool-Gómez E., López-Ramos M. Susceptibility to cardiac ischemia. reperfusion injury modulated by an estrogen derivative. African J. Pharm. Pharmacol. 2012;6:2493-2502.
- Figueroa-Valverde L., Hau-Heredia L., García-Cervera E., Pool-Gómez E., López-Ramos M., Vera-Escobedo M. Activity exerted by a naphthalene-oxirane derivative on the ischemia/reperfusion injury. Biom. Res. 2017;28:16-22.
- Bralet J., Didier J., Moreau D., Opie L., Rochette L. Effect of α-adrenoceptor antagonists (phentolamine, nicergoline and prazosin) on reperfusion arrhythmias and noradrenaline release in perfused rat heart. Br. J. Pharmac. 1985;84:9-18.
- Figueroa-Valverde L., Díaz-Cedillo F., García-Cervera E., Pool-Gómez E., Rosas-Nexticapa M., Hau-Heredia L, Eet al. Design and synthesis of new dihydrotestosterone derivative with positive inotropic activity. Steroids. 2015;95:39-50.
CrossRef - Sarabia-Alcocer B., Figueroa-Valverde L., Díaz-Cedillo F., Hau-Heredia L., Rosas-Nexticapa M., García-Cervera E., Pool-Gómez E., García-Martínez R., Zepeda-Acosta B. Activity Induced by a Naphthalene-Prazosin Derivative on Ischemia/Reperfusion Injury in Rats. Pharmacol. Pharm. 2014;5,1130-1142.
CrossRef - Okada T., Izawa N., Tai N. Comparison of the Effects of Nifedipine and Nisoldipine on Coronary Vasoconstriction in the Langendorff-Perfused Rat Heart. J. Cardiov. Pharmacol. 2000;35:145-149.
CrossRef - Rump A., Blazincic B., Klaus W. Effect of amrinone and milrinone on myocardial ischemia extent and infarct size in isolated rabbit hearts. Arzneimittelforschung. 1993;43:1262-1266.
- Pasdois P., Quinlan C., Rissa A., Tariosse L., Vinassa B., Costa A. D., et al. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving src-kinase, mito-KATP and ROS. Am. J. Physiol. Heart. Circ. Physiol. 2007;292:1470-1478.
CrossRef - Toombs C., McGee D. Myocardial Protective Effects of adenosine infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992;86:986-994.
CrossRef - Leprán I., Pollesello P., Vajda S., Varró A., Papp J. Preconditioning Effects of Levosimendan in a Rabbit Cardiac Ischemia-Reperfusion Model. J. Cardiov. Pharmacol. 2006;48:148-152.
CrossRef - Booth E., Obeid N., Lucchesi B. Activation of Estrogen Receptor-α Protects the in Vivo Rabbit Heart from Ischemia-Reperfusion Injury. Am. J. Physiol. Heart. Circ. Physiol. 2005;289:2039-2047.
CrossRef - He W., Wilder T., Cronstein B. Rolofylline an adenosine A1-receptor antagonist, inhibits osteoclast differentiation as an inverse agonist. Brit. J. Pharmacol. 2013;170:1167-1176.
CrossRef - Tikh E., Fenton R., Dobson J. Contractile effects of adenosine A1 and A2A receptors in isolated murine hearts. Am. J. Physiol. Heart Circ. Physiol. 2006;290:348-356.
CrossRef - Hayes J., Brunton L., Mayer S. Szokodi I., Kinnunen P., Tavi P., Weckstrom M., Toth M., Ruskoaho H. Selective Activation of Particulate CAMP-dependent Protein Kinase by Isoproterenol and Prostaglandin. J. Biol. Chem. 1980;255:5113-5119. 31. Evidence for Camp -Independent Mechanisms Mediating the Effects of Adrenomedullin a New Inotropic Peptide. Circulation. 1998;97:1062-1070.
CrossRef - Hocht C., Opezzo J., Gorzalczany S., Bramuglia G., Tiara C. Una aproximación cinética y dinámica de metildopa en ratas con coartación aórtica mediante microdiálisis. Rev. Argent. Cardiol. 1999; 67:769-73.
- Kato R., Foëx P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: An update for anesthesiologists. Canadian J. Anesth. 2002;49:777-791.
CrossRef - Salaris S., Babbs C., Voorhees W. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase: A potential new drug for the attenuation of ischemia/reperfusion injury. Biochem. Pharmacol. 1991;42:99-506.
CrossRef - Ray P., Maulik G., Cordis G., Bertelli A., Bertelli A., Das D. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free. Rad. Biol. Med. 1999;27:160-169.
CrossRef - Khan S., Salloum F. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J. Mol. Cell. Cardiol. 2006;41:256-264.
CrossRef - Rossoni G., Manfredi B., Colonna V., Bernareggi M., Berti F. The Nitroderivative of Aspirin, NCX 4016, Reduces Infarct Size Caused by Myocardial Ischemia-Reperfusion in the Anesthetized Rat. J. Pharmacol, Exp. Ther. 2001;297:380-387.
- Zhang L., Le C., Lautens M. The Use of Silyl Ketene Acetals and Enol Ethers in the Catalytic Enantioselective Alkylative Ring Opening of Oxa/Aza Bicyclic Alkenes. Agew. Chem. Inter. Ed. 2014;53:5951-5954.
- De-Faria R., Correia C. Synthesis of Indolizidines and Pyrrolizidines through the [2 + 2]Cycloaddition of Five-Membered Endocyclic Enecarbamates to Alkyl Ketenes. Unusual Regioselectivity of Baeyer−Villiger Ring Expansions of Alkyl Aza-Bicyclic Cyclobutanones. J. Org. Chem. 2002;67:3651-3661.
CrossRef - Chin S., An H., Chien H. Synthesis of Aza Bicyclic Enones via Anionic Cyclization: Application to the Total Synthesis of (-)-Brunsvigine. Org. Lett. 2001;3(14):2177-2179.
CrossRef - Rubio I., Ramire I., Islas I., Ceballos G. Testosterone metabolites mediate its effects on myocardial damage induced by ischemia/reperfusion in male Wistar rats. Steroids. 2013;78(3):362-369.
CrossRef - Chunyan H., Hongmei G., Wenjun Z., Herrmann J., Wang M. Testosterone-Down-Regulated Akt Pathway During Cardiac Ischemia/Reperfusion: A Mechanism Involving BAD, Bcl-2 and FOXO3a. J. Surg. Res. 2010;164:e1-e11.
CrossRef - Zhai P., Eurell T., Cotthaus R., Jeffery E., Bahr J., Gross D. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am. J. Physiol. Heart. Circ. Physiol. 2002;279:2766-2775.
CrossRef - Moraru I., Jones R., Popescu L., Engelman R., Das D. Prazosin reduces myocardial ischemia/reperfusion-induced Ca2+ overloading in rat heart by inhibiting phosphoinositide signaling. Biochim. Biophys. Acta (BBA)-Molr Cell. Res. 1995;1268:1-8.
- Zhu B., Simonis U., Cecchini G. Comparison of Pyrroloquinoline Quinone and or Metoprolol on Myocardial Infarct Size and Mitochondrial Damage in a Rat Model of Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2006;1:119-128.
CrossRef - Khaper N., Rigatto C., Seneviratne C., Li T., Singal P. Chronic Treatment with Propranolol Induces Antioxidant Changes and Protects Against Ischemia–Reperfusion Injury. J. Mol. Cell. Cardiol. 1997;29(12):3335-3344.
CrossRef - Nayler W., Ferrari R., Williams A. Protective effect of pretreatment with verapamil, nifedipine and propranolol on mitochondrial function in the ischemic and reperfused myocardium. Am. J. Cardiol. 19080;6:242-248.
CrossRef - Motro M., Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertens. 2001;37:1410-1413.
CrossRef - Sundberg S., Lilleberg J., Nieminen M., Lehtonen L. Hemodynamic and neurohumoral effects of levosimedan, a new calcium sensitizer, at rest and during exercise in healthy men. Am. J. Cardiol. 1995;75:1061-1066.
CrossRef - Huang M., Wu Y., Nguyen V., Rastogi S., McConnell B., Wijaya C., et al. Heart Protection by Combination Therapy with Esmolol and Milrinone at Late-Ischemia and Early Reperfusion. Cardiovasc. Drugs Ther. 2011;25:223-232.
CrossRef - Beller G., Smith T., Hood W. Effects of ischemia and coronary reperfusion on myocardial digoxin uptake. Am. J. Cardiol. 1975;36:902-907.
CrossRef - Liu G., Thornton J., Winkle D., Stanley A., Olsson R., Downey J. Protection Against Infarction Afforded by Preconditioning is Mediated by A1 Adenosine Receptors in Rabbit Heart. Circulation. 1991;84:350-356.
CrossRef - Massie B., O’Connor C., Metra M., Ponikowski P., Teerlink M., Cotter G., et al. Rolofylline, an Adenosine A1-Receptor Antagonist, in Acute Heart Failure. Engl. J. Med. 2010;363:1419-1428.
CrossRef - Poucher S., Keddie J., Singh P., Stoggall S., Caulkett P., Jones G., et al. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. Brit. J. Pharmacol. 1995;115:1096-1102.
CrossRef - Akbar M., Okajima F., Tomura H., Shimegi S., Kondo Y. A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase C and arachidonate release. Mol. Pharmacol. 1994;45:1036-1042.







